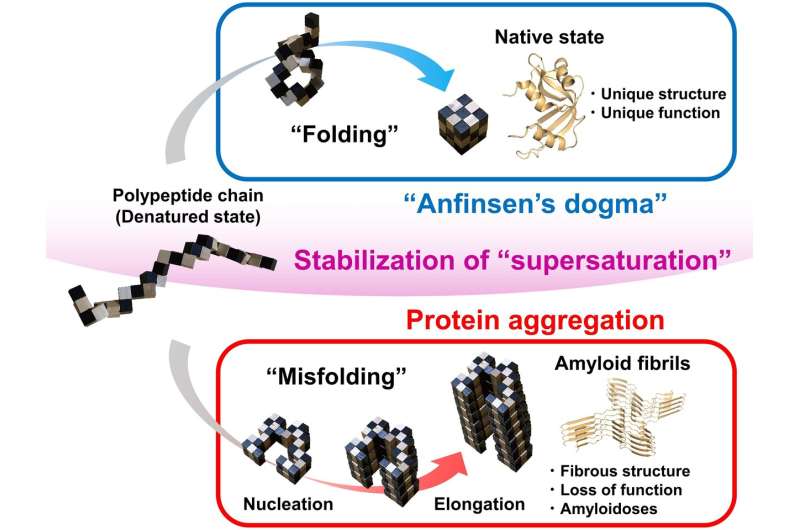
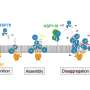
Fig. 1. Snake puzzle models of protein folding and amyloid fibril formation. Protein folding under Anfinsen’s dogma leads to a unique functional native structure. Protein misfolding out of Anfinsen’s dogma leads to amyloid structures. The two reactions are separated by the high free energy barrier of supersaturation. Once this barrier is broken by agitation or seeding, the equilibrium of folding and misfolding will be achieved. Credit: Osaka University
Correct, or native, protein folding is essential for correct protein function. Protein misfolding can lead to the formation of amyloid fibrils, and amyloidosis, which is implicated in various human neurodegenerative diseases, including Parkinson's, Alzheimer's, and Huntington's diseases. In this study Yuji Goto and colleagues describe, for the first time, a dynamic link between protein folding and misfolding, and the threshold that must be overcome for the formation of amyloid fibrils.
Technological advances are at the forefront of many scientific discoveries. The atomic structures of some amyloid fibrils were recently revealed as a result of advances in solid-state nuclear magnetic resonance and cryogenic electron microscopy. While an important step forward for the field, this development does not fully explain the determining factors of protein misfolding. How are folding and misfolding related? Can folding/unfolding and amyloid polymerization/depolymerization be explained by a single mechanism, and if so what might this look like? These are the questions that researchers at Osaka University sought to answer.
Summarizing their motivation for this work, senior author Masahiro Noji explains: "The thermodynamic hypothesis of protein folding, known as the "Anfinsen's dogma' describes that the native structure of a protein represents a free energy minimum determined by the amino acid sequence. However, this is not consistent with the misfolding of globular proteins to form amyloid fibrils." Therefore, Yuji Goto and colleagues set out to explore the link between protein folding and misfolding.
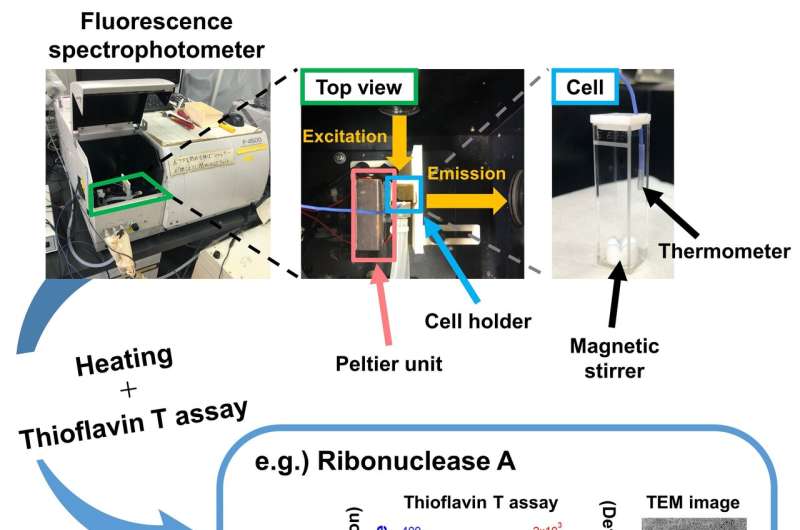
Fig. 2. Observation of heating-induced aggregation by simultaneous light scattering and thioflavin T fluorescence monitoring. (a) Experimental set-up for simultaneously monitoring light scattering and thioflavin T fluorescence under controlled heating and stirring. (b) Heating- and agitation-dependent amyloid formation of ribonuclease A. Credit: Osaka University
Although proteins perform their functions by folding to their native structures, as represented by Anfinsen's dogma, proteins often misfold to form amyloid fibrils, leading to amyloidosis. In their paper, the research team from Osaka University describe a general concept for the link between protein folding and misfolding.
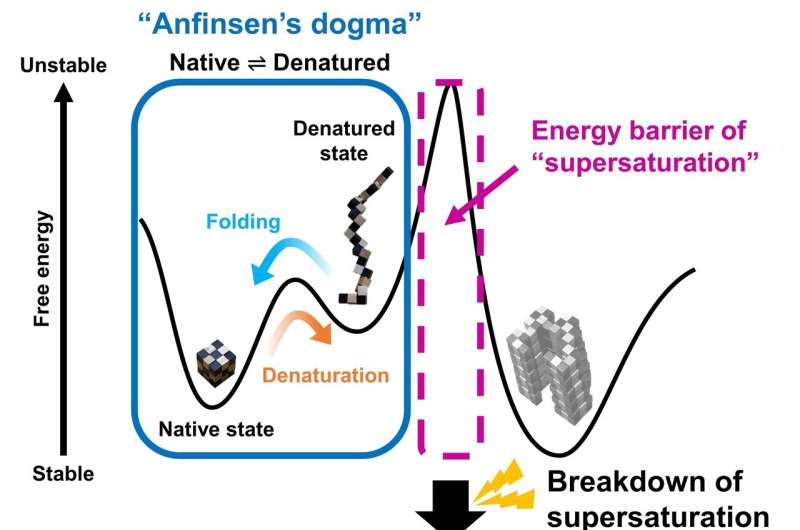
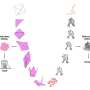
Fig. 3. Schematic representation of free energies before (a) and after (b) the linkage of folding and misfolding transitions. (a) A two-state transition independent of protein concentration persists. The high free energy barrier of supersaturation and the folding/unfolding transition between native (N) and unfolded (D) states is consistent with Anfinsen’s dogma. (b) Upon breaking supersaturation, by agitation, the folding/unfolding transition and the aggregation to polymeric states (P) enter an equilibrium, and the solubility of D determines the overall equilibrium. Credit: Osaka University
"The supersaturation barrier of a denatured protein separates protein folding and amyloid formation, and misfolding occurs when this barrier breaks down" corresponding author Yuji Goto says. "Our results show a clear link between correct protein folding, as defined by Anfinsen's dogma, and protein misfolding."
Supersaturation can be observed throughout nature in the formation of crystals, including those involved in ice formation. Here, the team at Osaka University show that supersaturation is fundamental to correct protein folding. The supersaturation barrier represents a novel concept that will advance the field of protein folding and contribute to the development of therapeutic strategies to prevent and treat amyloidosis, including those involved in neurodegenerative diseases.
More information: Masahiro Noji et al. Breakdown of supersaturation barrier links protein folding to amyloid formation, Communications Biology (2021). DOI: 10.1038/s42003-020-01641-6
Journal information: Communications Biology
Provided by Osaka University