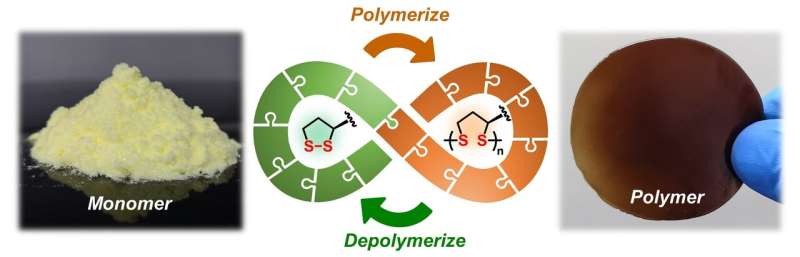
Scientists from the University of Groningen (The Netherlands) and the East China University of Science and Technology (ECUST) in Shanghai produced different polymers from lipoic acid, a natural molecule. These polymers are easily depolymerized under mild conditions. Some 87 percent of the monomers can be recovered in their pure form and re-used to make new polymers of virgin quality. Credit: Qi Zhang, University of Groningen
Plastics are among the most successful materials of modern times. However, they also create a huge waste problem. Scientists from the University of Groningen (The Netherlands) and the East China University of Science and Technology (ECUST) in Shanghai produced different polymers from lipoic acid, a natural molecule. These polymers are easily depolymerized under mild conditions. Some 87 percent of the monomers can be recovered in their pure form and re-used to make new polymers of virgin quality. The process is described in an article that was published in the journal Matter on 4 February.
A problem with recycling plastics is that it usually results in a lower-quality product. The best results are obtained by chemical recycling, in which the polymers are broken down into monomers. However, this depolymerization is often very difficult to achieve. At the Feringa Nobel Prize Scientist Joint Research Center, a collaboration between the University of Groningen and ECUST, scientists developed a polymer that can be created and fully depolymerized under mild conditions.
Perfect building block
"We found a way to produce polymers from the natural molecule lipoic acid in a very controlled way," explains Ben Feringa, Professor of Organic Chemistry at the University of Groningen. "It is a beautiful molecule and a perfect building block that was created by nature." The molecule has a ring structure that includes a sulfur-sulfur bond. When this bond is broken, the sulfur atoms can react with those of another monomer. "This process was known before, but we managed to find a way to control it and to create long polymers."
Elastic material
The molecule also has a carboxyl group, which readily reacts with metal ions. These can crosslink the polymers, which results in an elastic material. By dissolving the molecule in water with sodium hydroxide and then evaporating the water, a firmer polymer film is produced through ionic bonds. As the polymerization is achieved through reversible bonds, the material is also self-healing, explains Feringa: "When it is cut, you can simply press the ends together and they will reconnect in a few minutes."
Dr. Qi Zhang, first author of the paper in Matter. Credit: Qi Zhang, University of Groningen
Reversible polymerization
Most of the work in the Materials paper was carried out by Qi Zhang, first as a Ph.D. student at ECUST in Shanghai and later as a postdoctoral researcher at the University of Groningen. "Lipoic acid is a natural small molecule with an elegant structure,' he says. 'We didn't have to do any tedious re-designing of the monomer to achieve a fully reversible polymerization." Simply exposing the polymers to sodium hydroxide dissolves the polymers into monomers. "By adding a little acid, the monomers precipitate and can be recovered. The quality of these recycled monomers is identical to that of the original material."
Recycling without loss of quality
"Our experiments show what is possible with these monomers," adds Feringa. "We can even recycle the material into monomers several times, without loss of quality." However, industrial applications of this new polymer are a long way off. Feringa says, "This is a proof of principle. We are conducting experiments now to create polymers with new functionalities and to better understand the polymerization and depolymerization processes." Furthermore, although 87 percent of the monomers can already be recovered, the scientists want to get as close to a hundred percent as possible. "Our experiments show that we can produce, in a controlled fashion, hard and soft, elastic polymers that can be fully depolymerized," Feringa sums up. "This molecule is really very promising."
More information: Da-Hui Qu et al, Dual closed-loop chemical recycling of synthetic polymers by intrinsically reconfigurable poly(disulfides) Matter 4 February 2021. DOI: 10.1016/j.matt.2021.01.014
Journal information: Matter
Provided by University of Groningen