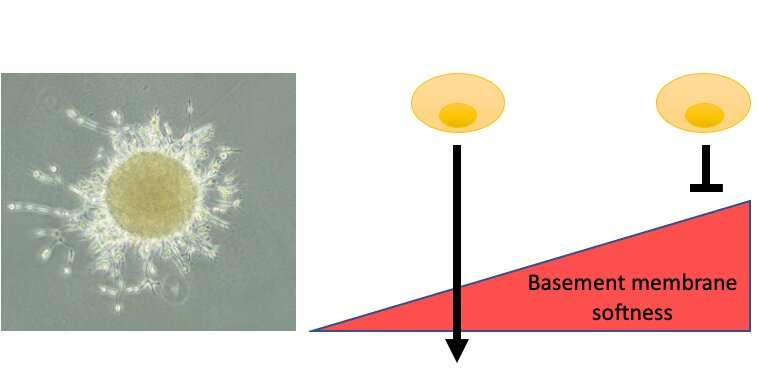
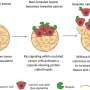
LEFT: Spheroid of breast cancer cells breaking through basement membrane to invade into the surrounding area. This ability is determined by the ratio of netrin-molecules present in the basement membrane composition (image from Erler lab). RIGHT: Schematic to explain the impact of basement membrane softness on cancer cell’s ability to cross vessels. Credit: University of Copenhagen
Researchers from Erler Group at the Biotech Research & Innovation Centre (BRIC) in Copenhagen have discovered that the rigidity of a thin membrane structure encompassing cells and lining all vessels regulates how easily cancer cells can breach tissues to spread through the body, and is thus a key determinant of cancer patient survival. The results are published in Nature Materials today.
The researchers analyzed cells, mouse models, and human patient samples using biochemical, mathematical, and biophysical methods. They identified a protein present in the mesh-like membrane structure (the basement membrane) associated with tumor and vessel softness, and good survival of cancer patients. The researchers tested if removing this protein from the basement membrane would enhance the spread of cancer, which it did, and if supply of this protein would reduce cancer spread, which it did. They proceeded to show that the levels of this protein (netrin-4) already present in the basement membrane of organs may determine cancer spread even before cancer develops, in several cancer types.
"These are extremely exciting findings that open up the possibility to predict which organs a person's cancer most probably spread to before they even have cancer. This information therefore has the potential to guide and improve cancer patient treatment and care," said Professor Janine Erler, senior researcher on the paper.
In their study, the researchers could show for the first time the impact of basement membrane composition on its mechanical properties thereby affecting cancer cell transmigration over this protein border within the inter-cellular space. They identified the secreted protein netrin-4 to open nodes inside the basement membrane network, which simultaneously softens the basement membrane and increases its mesh (pore) size. This unexpected finding highlights that cancer cell movement is dominantly controlled by the basement membrane stiffness and pore size plays an underpart. Thus, the more netrin-4 inside the basement membrane of the primary tumor or inside blood vessels within organs prone to metastasis, the less metastasis, impacting on patient survival. Moreover, they demonstrate for the first time that the mechanical properties of the basement membrane independent of cancer-related modifications are a pivotal determinant of cancer patient survival.
"We were incredibly excited to find a mechanistic explanation for our observations where the theoretical modeling closely matched our experimental data. We could show that the more netrin-4 molecules present, the softer the basement membrane and the more difficult for a cell to traverse this membrane thereby keeping cells contained in one area. We are currently exploring the therapeutic and diagnostic potential of our findings. Our study is a result of a huge collaboration effort from researchers in Denmark, Sweden, Germany, the UK, and Belgium, spanning many disciplines, which has been key to obtaining the exciting results," added Dr. Raphael Reuten, lead researcher on the study.
Historically, there has been much focus on the stiffness of the extracellular matrix that lies outside of cells and the influence on cancer progression. However, there have not been studies investigating the influence of basement membrane stiffness. Moreover, studying the impact of mechanical properties of the basement membrane on cell invasion and cancer metastasis has not been possible so far. The mechanistic insights into netrin-4 activity inside basement membranes has enabled the researchers to bridge this gap of knowledge and present new opportunities to study basement membrane stiffness in a broad range of biological processes.
More information: Raphael Reuten et al, Basement membrane stiffness determines metastases formation, Nature Materials (2021). DOI: 10.1038/s41563-020-00894-0
Journal information: Nature Materials
Provided by University of Copenhagen