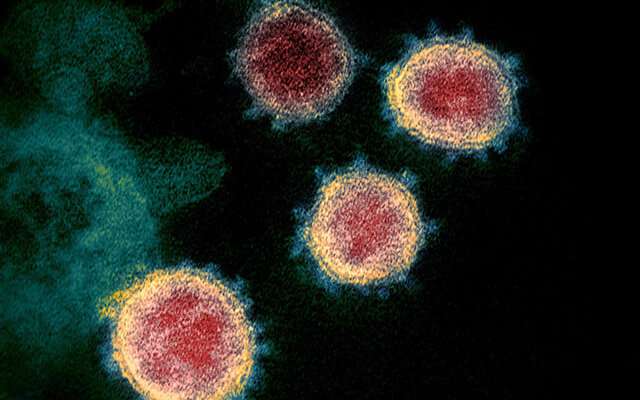
A colorized scanning electron micrograph of the SARS-CoV-2 virus. Credit: NIAID
A group of scientists led by EMBL's Mikhail Savitski, Nassos Typas, and Pedro Beltrao, and collaborator Steeve Boulant at Heidelberg University Hospital, have analyzed how the novel coronavirus affects proteins in human cells. They identified several human proteins as potential drug targets to prevent viral replication.
The researchers used a biophysical method called thermal proteome profiling (TPP) to gain a comprehensive overview of which human proteins are functionally altered during SARS-CoV-2 infection. TPP monitors protein amounts and denaturation temperatures—the points at which proteins heat up so much that they lose their 3-D structure. A shift in denaturation temperature indicates that a particular protein has undergone a functional change upon infection, possibly due to the virus hijacking the protein for use in its own replication.
The scientists observed that infection with SARS-CoV-2 changed the abundance and thermal stability of hundreds of cellular proteins. This included thermal stability changes in proteins required to maintain the cytoskeleton—a protein network that maintains cell shape and stability—and a group of proteins called heat shock chaperones: proteins that take care of unfolded or misfolded proteins and help them to maintain their 3-D structure.
Having identified candidate proteins and cellular processes that SARS-CoV-2 may hijack to promote its replication, the scientists used drugs to inhibit some of the host proteins that SARS-CoV-2 appeared to be exploiting. This resulted in a strong reduction in viral replication in the presence of two of the tested compounds, demonstrating their potential as antiviral therapeutics. The scientists believe that other proteins they identified could be targeted in a similar way to block SARS-CoV-2 proliferation.
The study was a collaborative effort, involving the Savitski team and Typas group in EMBL Heidelberg's Genome Biology Unit, EMBL's Proteomics Core Facility, the Beltrao group at EMBL's European Bioinformatics Institute (EMBL-EBI), the Boulant lab and colleagues from Heidelberg University Hospital, the German Cancer Research Center (DKFZ), and the Technical University of Munich. As well as gaining new insights into SARS-CoV-2 biology, the researchers were also able to expand the scope of the TPP methodology by adapting it to work with infectious samples under high biosafety conditions. This enables them to quickly analyze the effects of dangerous pathogens on the cell's proteins.
More information: Joel Selkrig et al. SARS-CoV-2 infection remodels the host protein thermal stability landscape. Molecular Systems Biology, published online 16 February 2021. DOI: 10.15252/msb.202010188
Journal information: Molecular Systems Biology
Provided by European Molecular Biology Laboratory