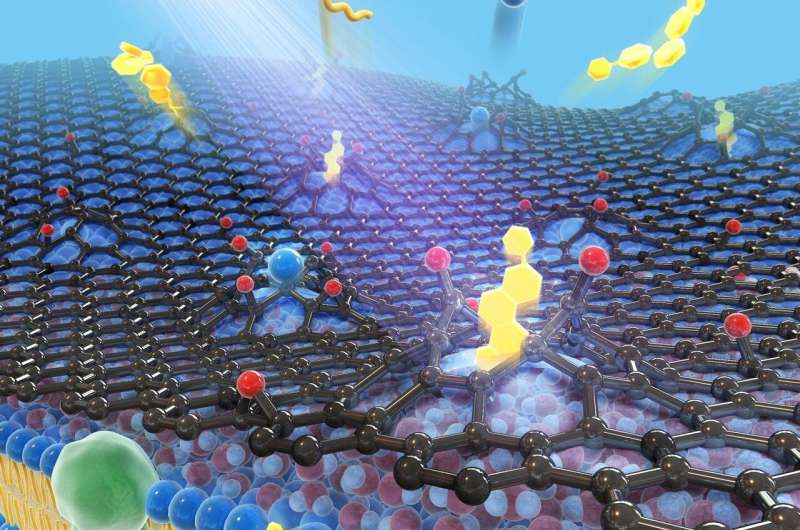
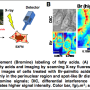
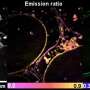
Understanding in detail the molecular structure of the cell membrane is important for learning more about the underlying cellular mechanisms of diseases. New techniques are needed to look at these miniscule structures in high resolution and with high accuracy Credit: Nature Methods
With every passing day, human technology becomes more refined and we become slightly better equipped to look deeper into biological processes and molecular and cellular structures, thereby gaining greater understanding of mechanisms underlying diseases such as cancer, Alzheimer's, and others.
Today, nanoimaging, one such cutting-edge technology, is widely used to structurally characterize subcellular components and cellular molecules such as cholesterol and fatty acids. But it is not without its limitations, as Professor Dae Won Moon of Daegu Gyeongbuk Institute of Technology (DGIST), Korea, lead scientist in a recent groundbreaking study advancing the field, explains: "Most advanced nanoimaging techniques use accelerated electron or ion beams in ultra-high-vacuum environments. To introduce cells into such an environment, one must chemically fix and physically freeze or dry them. But such processes deteriorate the cells' original molecular composition and distribution."
Prof. Moon and his team wanted to find a way to avoid this deterioration. "We wanted to apply advanced nanoimaging techniques in ultra-high-vacuum environments to living cells in solution without any chemical and physical treatment, not even fluorescence staining, to obtain intrinsic biomolecular information that is impossible to obtain using conventional bioimaging techniques," Dr. Heejin Lim, a key member of the research team, explains. Their novel solution is published in Nature Methods.
Their technique involves placing wet cells on a collagen-coated wet substrate with microholes, which in turn is on top of a cell culture medium reservoir. The cells are then covered with a single layer of graphene. It is the graphene that is expected to protect the cells from desiccation and cell membranes from degradation.
Through optical microscopy, the scientists confirmed that, when prepared this way, the cells remain viable and alive up to ten minutes after placing in an ultra-high-vacuum environment. The scientists also performed nanoimaging, specifically, secondary ion mass spectrometry imaging, in this environment for up to 30 mins. The images they captured within the first ten minutes paint a highly detailed (submicrometer) picture of the true intrinsic distribution of lipids in their native states in the cell membranes; for this duration, the membranes underwent no significant distortion.
With this method too, however, a cascade of ion beam collisions at a point on the graphene film can create a big enough hole for some of the lipid particles to escape. But while this degradation to the cell membrane does occur, it is not significant within the ten-minute window and there is no solution leakage. Further, the graphene molecules react with water molecules to self-repair. So, overall, this is a great way to learn about cell membrane molecules in their native state in high resolution.
"I imagine that our innovative technique can be widely used by many biomedical imaging laboratories for more reliable bioanalyses of cells and eventually for overcoming complex diseases," says Prof. Moon.
Will this innovation become the norm? Only time will tell!
More information: Heejin Lim et al, Mass spectrometry imaging of untreated wet cell membranes in solution using single-layer graphene, Nature Methods (2021). DOI: 10.1038/s41592-020-01055-6
Journal information: Nature Methods
Provided by DGIST (Daegu Gyeongbuk Institute of Science and Technology)