January 18, 2021 feature
Interstellar chemistry: low-temperature gas-phase formation of indene in the interstellar medium
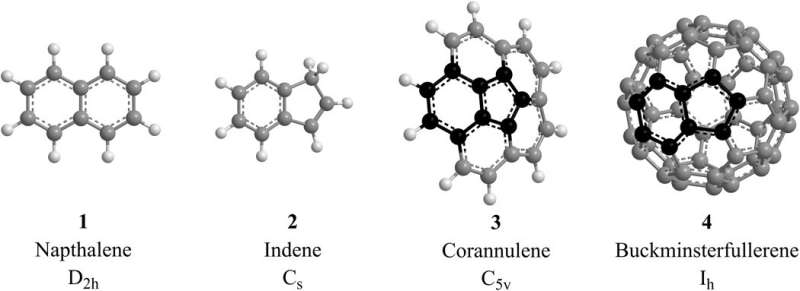
The interstellar medium and combustion systems contain polycyclic aromatic hydrocarbons (PAHs) as fundamental molecular building blocks that form fullerenes and carbonaceous nanostructures. However, researchers have yet to investigate and understand aromatic molecules carrying five-membered rings that form the essential building blocks of nonplanar polycyclic aromatic hydrocarbons (PAHs), which eventually lead to the formation of interstellar grains or carbonaceous cosmic dust. In a new report now published in Science Advances, Srinivas Doddipatla and a team of scientists in chemistry, physics and astronomy in the U.S. and Russia explored the concept with crossed molecular beam experiments, electronic structure calculations and astrochemical modeling. The work revealed an unusual pathway to form indene (C9H8)—a prototype aromatic molecule with a five membered ring. The mechanism was based on a barrierless biomolecular reaction that involved the simplest organic radical – methylidyne (CH) and styrene (C6H5C2H3) via a hitherto elusive methylidyne addition-cyclization-aromatization (MACA) mechanism. The work offers a new concept on the low-temperature chemistry of carbon found in the galaxy.
Interstellar chemistry
In this work, Doddipatla et al. revealed the synthesis of indene molecules based on elementary reactions between the simplest organic radical methylidyne with styrene molecules under single collision conditions. According to a hypothesis proposed by Léger and Puget in 1984, polycyclic aromatic hydrocarbons (PAHs) were assumed to be made of fused benzene rings—to form the missing link between small carbon molecules and carbonaceous nanoparticles or interstellar grains. The PAHs alongside their hydrogenated, alkylated, protonated and ionized counterparts are typically associated with diffuse interstellar bands (DIBs) from the visible to the near infrared and the unidentified infrared (UIR) emission range.
The compounds encompass about 20 percent of the carbon budget within the galaxy including carbonaceous chondrites (meteorites) such as Murchison, Allende, and Orgueil to advocate for a circumstellar origin of aromatics in carbon-rich asymptotic giant branch stars (AGB). The PAHs also constituted descendent planetary nebulae of AGB stars based on hydrogen abstraction—carbon addition (HACA) sequences. Once formed, however, interstellar PAHs are swiftly destroyed by galactic cosmic rays, photolysis and shock waves with lifetimes of only 108 years. As a result, PAHs should neither exist in the interstellar medium nor in meteorites and therefore their ubiquity presents a paradox in astrophysics. This inconsistency can be solved by assuming the existence of a hitherto elusive low-temperature route for the rapid growth of PAHs in the interstellar medium to overcome their destruction. The identification of such low-temperature pathways will help untangle the origin of PAHs containing five-membered rings such as indene at the most fundamental, microscopic level.
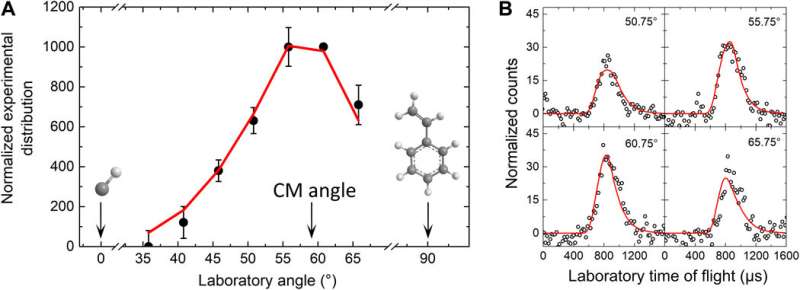
The experiments
The team combined crossed molecular beam reactive scattering experiments with electronic structure calculations and astrochemical studies to understand the unexpected gas-phase chemistry initiated by a single collision event. Such phenomena functioned at temperatures as low as 10 K present in molecular clouds such as the Taurus molecular cloud (TMC-1) and the Orion molecular cloud. The hitherto unknown methylidyne addition-cyclization-aromatization (MACA) mechanism explored in this work represented a barrierless path to form indene within the molecular clouds via rapid gas-phase chemistry. The findings disputed established paradigms by suggesting that low temperature initiated the formation of indene, the very first aromatic molecules in the interstellar medium. The carbon backbone of indene also represented a fundamental molecular building block of nonplanar PAHs and may lead to interstellar fullerene (C60, C70) formation.
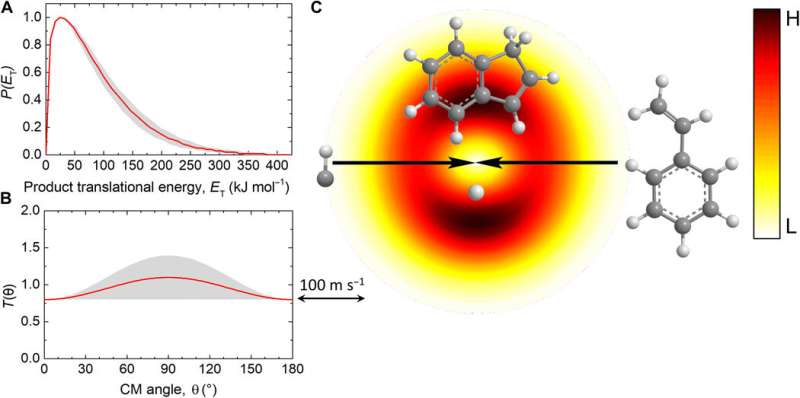
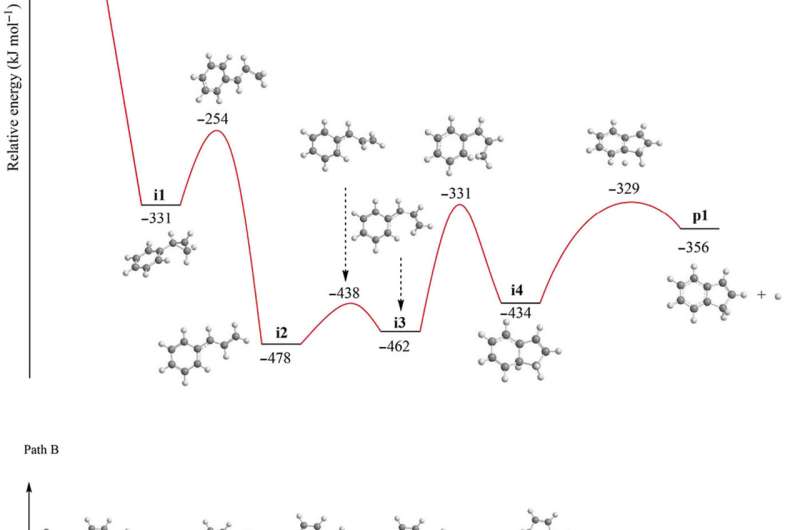
Mechanisms of indene formation
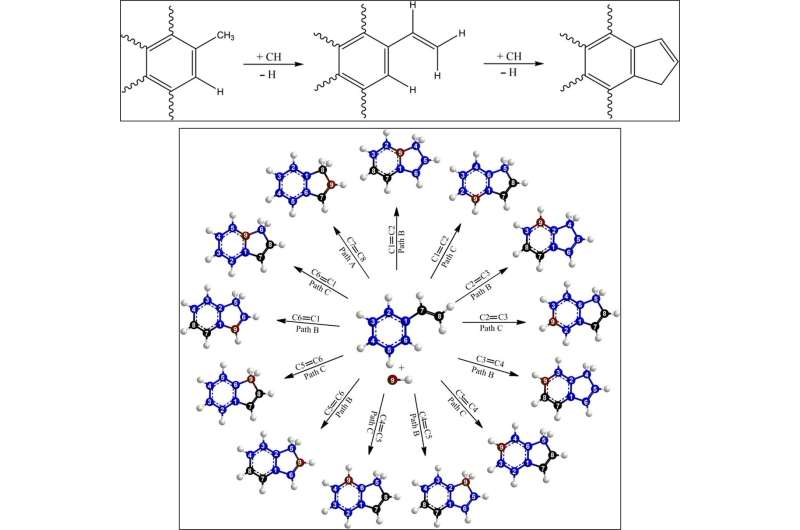
Since elementary reactions of the methylidyne radical and styrene in the gas phase formed the indene molecule, the team combined these findings with simulations and statistics to propose the underlying reaction mechanism. The computations revealed how the methylidyne radical could be added barrierlessly either to the π electron density of the carbon-carbon double bond of the vinyl moiety (C2H3) or to the aromatic ring. During methylidyne addition to the vinyl moiety, they observed a series of thermodynamically stable reactions, followed by cyclization reactions to emit atomic hydrogen accompanied by indene, in an overall exoergic reaction. The alternative methylidyne addition reaction to the benzene moiety was comparatively more complex. After identifying six feasible reaction pathways to form the expected products, the team explored the Rice-Ramsperger-Kassel-Marcus (RRKM) chemical kinetics theory to predict the dominant reaction pathway to form indene. They showed how indene could not be formed by itself in the absence of hydrogen atoms originating from the methylidyne reactant.
Astrochemical models
Using astrochemical models, Doddipatla et al. next studied how these lab outcomes could be transferred to the interstellar medium. The experimental findings provided vital criteria for the reaction to occur in low-temperature environments such as molecular clouds, where both methylidyne and styrene reactants exist. For instance, the methylidyne radicals can be generated within the internal ultraviolet (UV) photon field deep within molecular clouds. The scientists therefore conducted astrochemical simulations for the cold Taurus molecular cloud (TMC-1) using the Nautilus V1.1 code, to explore the efficiency of the MACA mechanism during indene formation in the interstellar medium. The results showed that while astronomically detecting indene in TMC-1 was challenging, it was technically feasible to conduct the experiments with high-spectral resolution and high sensitivity by using the Robert C. Byrd Green Bank Telescope (GBT) or the Atacama Large Millimeter/submillimeter Array (ALMA).
Outlook
In this way, Srinivas Doddipatla and colleagues combined crossed molecular beams, electronic structure and astrochemical modeling to reveal the potential formation of indene across hot, carbon-rich stars and planetary nebulae, as well as in cold molecular clouds. The mechanism involved a simple, barrierless reaction based on the simplest organic radical methylidyne with styrene. The work represented an important step to understand the fundamental chemical processes forming indene and nonplanar polycyclic aromatic hydrocarbons (PAHs) in low-temperature environments in deep space. Given the crucial role that nonplanar PAHs play in the formation of carbonaceous cosmic dust particles commonly known as interstellar grains during the chemical evolution of the universe, understanding the elementary steps leading to cosmic dust particle formation will enhance the astrochemical awareness of our galaxy.
More information: Doddipatla S. et al. Low-temperature gas-phase formation of indene in the interstellar medium, Science Advances, DOI: 10.1126/sciadv.abd4044
Kim H. S. et al. Single photon infrared emission spectroscopy of the gas phase pyrene cation: Support for a polycyclic aromatic hydrocarbon origin of the unidentified infrared emission bands, Physical Review Letters. doi: doi.org/10.1103/PhysRevLett.86.5691
Zenobi R. et al. Spatially resolved organic analysis of the allende meteorite. Science, 10.1126/science.246.4933.1026
Journal information: Physical Review Letters , Science , Science Advances
© 2021 Science X Network




















