Greener chemistry through new approach to catalysis
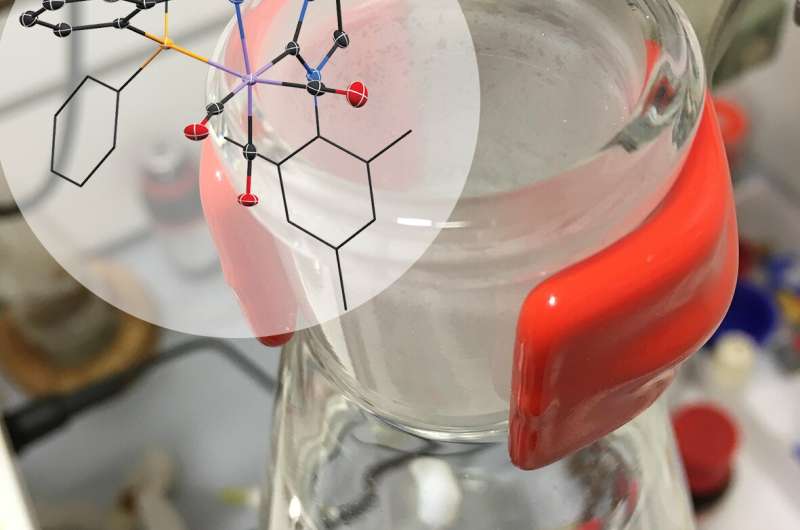
Researchers at Delft University of Technology (TU Delft) have developed a catalyst that is effective in negligible amounts. Due to its form and durability, the catalyst lasts much longer in reactions, saving a great deal of energy, preventing waste and reducing costs. The results have been published in Nature Communications.
TU Delft researcher Evgeny Pidko and his group take an almost holistic approach to chemical reactions using catalysts. These substances play an important role in lowering the activation barriers for chemical reactions.
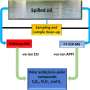
The catalyst is present in the reaction mixture but is not part of the chemical reaction equation. So in theory, catalysts should be able to work forever. In practice, however, chemists see that they become less active over time. This means that a large amount of catalyst is needed for the reaction process to run smoothly. The catalyst then has to be removed from the reaction mixture, which is an expensive and polluting process. For the pharmaceutical and fine chemical industry, it is not uncommon that producing one kilogram of the required product results in around fifty kilos of waste products, so it is a matter of some urgency to make these chemical processes more sustainable.
Birth and death
The most important question facing Pidko's team is: Under what circumstances are catalysts active, and why do they decay? Pidko: "What we are actually doing is following a catalyst from its birth to its death." This research has now led to the discovery of a new catalyst that is exceptionally efficient. Even using 'homeopathic' amounts of the catalyst is enough for the reaction to run well. For their research, the chemists used a manganese catalyst that hydrogenates molecules, meaning that a double bond in the molecule is broken and hydrogen takes this place in the molecule. This reaction is mainly used to solidify oils and fats.
Pidko: "To make the catalyst more effective, we played around to create more bonding points per molecule. We noticed that all of a sudden the reaction went a lot better." Using spectroscopy, the researchers observed that the new catalyst has ideal properties. In its activated state, it opens up to promote the required reaction. In its resting state the catalyst closes its pincer-like arms, protecting its active part. The research by Pidko's team shows that the catalyst can be used for up to 200,000 'cycles." Pidko says, "Even at temperatures of 120 °C it maintains its integrity. The efficacy of this catalyst is very unique." Rather than the usual 1000 ppm (parts per million), only 5 ppm of the new catalyst is needed. Using such minimal quantities means that the purification step that is usually needed can be skipped.
Greening the chemical industry
This development is an important step in the greening of the chemical industry, although it cannot be used right away in existing chemical processes, as further research is required. What makes this study particularly innovative in the field is the approach taken to catalysts. Pidko concludes, "Meticulously following the entire life cycle has given us a better understanding of catalysts and we hope we can use this approach to develop more of these 'homeopathic' catalysts, which could prove revolutionary for the chemical industry."
More information: Wenjun Yang et al. Robust and efficient hydrogenation of carbonyl compounds catalysed by mixed donor Mn(I) pincer complexes, Nature Communications (2021). DOI: 10.1038/s41467-020-20168-2
Journal information: Nature Communications
Provided by Delft University of Technology