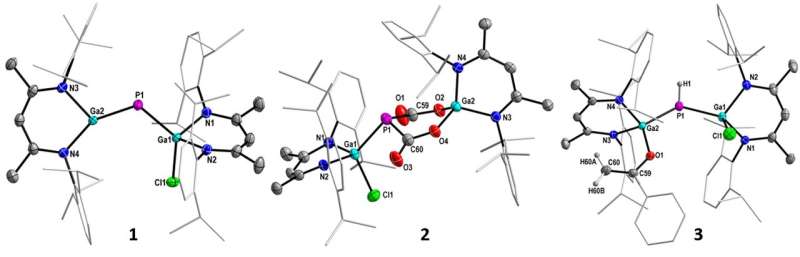
Structures of the gallaphosphene (1) and the complex of CO2 (2) and acetophenone (3). (c) UDE/S.Schulz
Group 13/15 heteroalkenes RMER' with M-E double bonds (M = B-Tl; E = N-Bi) offer promising potential for bond activation reactions, but they are difficult to prepare. A team led by CENIDE professor Stephan Schulz now describes new synthetic methods for group 13 metallapnictenes in no less than three articles in the journal Angewandte Chemie. They allow for the preparation of preparative amounts as a basis for systematic reactivity studies.
It was shown that the gallaphosphene L(CL)Ga=P-GaL (L =β-diketiminate) not only selectively activates the C(sp3)-H bonds of acetone and acetophenone, but also CO2. The latter reaction is even completely reversible in this process, and thus the bound CO2 can be re-released with reversion of the gallaphosphene at 90 °C.
"I would never have thought that such a reversible reaction, that reveals new options in CO2 storage, was possible. Together with CH activation, the supreme discipline in organometallic chemistry, this results in a wide range of options—including catalytic reactions," Schulz says.
More information: Bin Li et al. Synthesis and Reactivity of Heteroleptic Ga‐P‐C Allyl Cation Analogues, Angewandte Chemie International Edition (2020). DOI: 10.1002/anie.202012595
Julia Krüger et al. From π‐Bonded Gallapnictenes to Nucleophilic, Redox‐Active Metal‐Coordinated Pnictanides, Angewandte Chemie International Edition (2020). DOI: 10.1002/anie.202013618
Stephan Schulz et al. Multi‐talented gallaphosphene for Ga–P–Ga heteroallyl cation generation, CO2 storage and C(sp3)–H bond activation, Angewandte Chemie International Edition (2020). DOI: 10.1002/anie.202014381
Journal information: Angewandte Chemie , Angewandte Chemie International Edition
Provided by Universität Duisburg-Essen