January 5, 2021 report
Full mitochondrial control for the ultimate anticancer biohack
Insofar as variants for mitochondrial disease are supposed to be rare in the genome, don't think for even a minute that it can't happen to you. In fact, the closer one looks at the full mitonuclear genomes of normal folks, the more one realizes that no one is actually normal—we are all, shall we say, temporarily asymptomatic.
But in the fullness of time, many asymptomatics develop the hallmarks of mitochondrial disease. While mitochondrial underperformance is ultimately behind many specific disease processes like the accumulation of unburnt fatty acids in fatty liver disease, or the clogging debris in degenerating tubules in renal disease, cancer is the entropic cellular eventuality for which we must all prepare. Depending on which organ, and which kind of tumor, cancer can be both a big bang and heat death of our existence—and both are controlled by mitochondrial energy.
Fully aware of these universal truths, researchers have long sought ways to control the spread of cancer by limiting specific mitochondrial activities. In other words, to curtail energetic and synthetic processes just enough to block exuberant replication and motility of cancer cells without wiping out our normal, less proliferative and lethargic cells. One way to do this was recently suggested by researchers from Sichuan University in Chengdu. The results were published in Advanced Science.
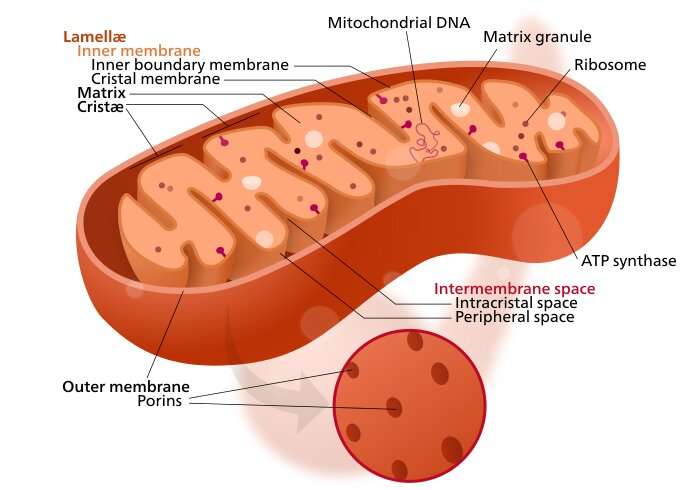
Their idea was to target an elusive pore complex found in the inner mitochondrial membrane known as the MPTP, for "mitochondrial membrane permeability transition pore." At this point, all the neurochemists should be up in arms because the acronym MPTP is already taken by a molecule by the name of 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine. This is the well-known prodrug to the neurotoxin MPP+, which causes permanent symptoms of Parkinson's. Although the actual protein identities of the mitochondrial MPTP have still not been entirely verified, it has been experimentally found to contain a pore that allows entry of molecules less than 1.5kDa, which corresponds to a diameter of ≈3 nm.
When enough pores are toggled into the open state from cellular stress or other pathology, the mitochondria will swell and cell death via necrosis quickly follows. If no pores are open, anticancer drugs like doxorubicin, which could potentially tamp down overactive mitochondria, can't get in. In order to gain control over the MPTP, the researchers turned to a magic ingredient known to affect permeability of the pore—licorice. Real licorice, as opposed to the fake-news substitutes peddled by most candy outlets, contains all the cortisol-inhibiting, potassium-wasting glycyrrhetinic acid (GA) your kidneys can handle.
How much GA, exactly, are talking about? Fortunately—or unfortunately, as the case may be—we already have an upper bound from actual toxicology reports from self-enrolling test subjects. For example, one man who willingly lunched on 2 lbs of real licorice candy every day, persisted in his habit for three weeks before falling ill. Once enzymatically relieved of its supernumerary sugar groups, the cholesterol-like GA blocks the breakdown of cortisol, causing the patient to promptly urinate away all their potassium. The secret sauce is that GA also acts via the respiratory chain to generate hydrogen peroxide, which then opens up MPTPs.
The researcher's strategy was to deliver a two-in-one blow by combining GA and doxorubicin on a nanoparticle shell, with a TPP‐doxorubicin combo in the core. TPP, or triphenyl phosphonium, is a lipophilic cation that can help to electrostatically shuttle compounds across the hard barrier of the negative mitochondrial membrane potential ΔΨ, typically hovering between -150 and -180 mV). The plan worked, and the nanoparticles successfully inhibited the growth of primary lung tumors, and also suppressed their metastases.
Targeting the MPTP is not the only way to go as far as stopping cancer. Other recent research has suggested that inhibiting the mitochondrial RNA polymerase (POLRMT) kills several tumor cell lines but is not cytotoxic to normal but active human cell types like hepatocytes or peripheral blood mononuclear cells. Researchers found that normal cytosolic ribosomes were unaffected by the POLRMT inhibitor, while the mitoribosomes were specifically depleted, consistent with a lack of transcription of the mitochondrial-encoded rRNA subunits. Importantly, the inhibitor did not interfere with other RNA polymerase needed in the nucleus of cells.
This kind of specificity would be quite handy as a counterbalance to new mitochondrial transplant therapies now proposed as treatments for various ailments. Whereas the concern would be creating new cancers from the extra mitochondrial supply, artfully applied inhibitors might short circuit such risks. This is all quite timely and convenient, because new hardware advancements in delivering the goods via mitochondrial replacement therapies are now coming into view. A couple early examples, the so-called photothermal nanoblade and the biophotonic laser-assisted cell surgery tool (BLAST) technologies, initially looked promising after they successfully transferred isolated mitochondria into osteosarcoma cells. However, they were laborious and low-throughput, and did not always meet the goal of resetting cell metabolomes.
Enter the new and improved mitochondrial uploader—the
MitoPunch. This pressure-driven device uses tiny mechanical plungers to deliver much larger cargoes using massively parallel arrays into various kinds of cells. The plunger deforms a pliable polydimethylsiloxane (PDMS) reservoir containing isolated mitochondria and propels through a porous membrane containing numerous 3-μm-diameter holes and into cell cytoplasm. The schema would be to take out some cells, mitopunch them, and then put them back in strategic places. One might even envision future refinements of the device that could be introduced via catheters in the circulatory system to reach targets deep in the heart, lung, muscle or even the brain.
The natural phenomenon that underlies the utility of such mitochondrial manipulations is the remarkable ability of the mitochondrial network to continually remodel itself through fusion and fission events through which mitochondrial RNA granules are processed and exchanged. The newly introduced recruits would not only be expected to participate in these events, but now, scientists can even watch them. For example, researchers from the Laboratory of Experimental Biophysics at the EPFL have recently built a live-cell, super-resolution microscope that can directly image newly minted mitochondrial RNA granules.
Incredibly, they found that what the RNA granules did, the whole network did. In other words, they could predict when the network would bifurcate or fuse based on what the RNA granules inside were doing. They even went so far as to call the coordinated display a fluid condensate. Furthermore, they could control the granules with specific inhibitors. Although we are still a ways from full mitochondrial control, these developments suggest continued promise and progress.
More information: Xi Lin et al. Targeting the Opening of Mitochondrial Permeability Transition Pores Potentiates Nanoparticle Drug Delivery and Mitigates Cancer Metastasis, Advanced Science (2020). DOI: 10.1002/advs.202002834
© 2021 Science X Network