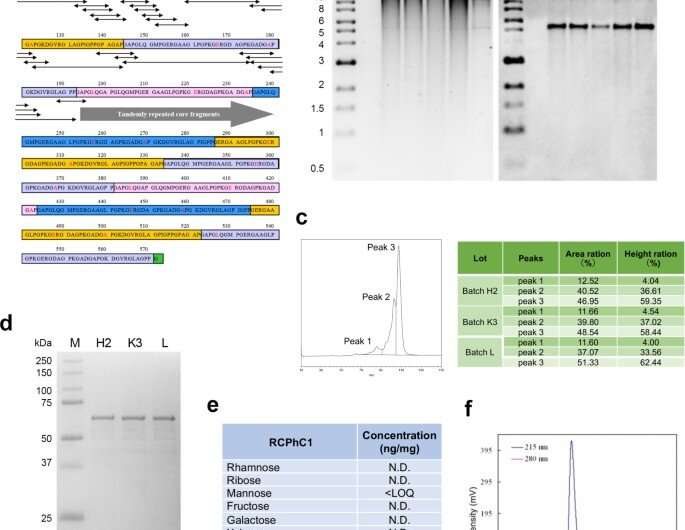
Molecular design and synthesis of recombinant polypeptide RCPhC1. (a) The peptide sequence of RCPhC1 shows Gly-Xaa-Yaa repeats with amino acid substitutions (red). All peptide fragments (minimum penta sequences) containing the amino acid substitutions (double head arrows) were matched with human collagen sequences. (b) Genomic DNA stability in the working cell bank. After each of four consecutive fragmentations, genomic DNA was isolated (lanes 1–4) and compared with the original genomic DNA (lane 5). The right panel, Southern blot hybridized with the AOX1 promoter probe. (c) Reproducibility was analyzed by liquid chromatography. The reverse-phase profile showed three peaks, which were consistently identified in three different batches (H2, K3, and L). (d) Reproducibility was also evaluated by SDA-PAGE. The single band of three different batches also suggested the absence of post-translational modification. (e) The absence of post-translational glycosylation. (f) Gel permeation chromatography confirmed the high purity of RCPhC1. Credit: Nature Communications Materials, doi: 10.1038/s43246-020-00089-9
The current gold standard for bone grafting surgery includes autografts and allografts, although a growing demand exists to develop synthetic biomaterials for enhanced bio-integration in bone tissue engineering. In a new report now published on Nature Communications Materials, Hideo Fushimi and a research team in bioscience and engineering, and reconstructive biotechnology in Japan and the U.S., developed a biodegradable scaffold material using recombinant proteins or polypeptides as a source of hydrogel-based graft materials. The team used human type I collagen alpha 1 chain (abbreviated RCPhC1) as a source to develop the recombinant polypeptide and demonstrated the flexibility of the material to engineer ideal characteristics for bone grafts. The team also developed RCPhC1 bone grafts using a highly scalable, streamlined production protocol for the robust generation of mature bone tissue in the lab. The bone graft completely resorbed after tissue regeneration in a preclinical animal model for effective biological integration.
Bone tissue engineering with biomimetic, synthetic bone grafts
In this work, Fushimi et al. developed a synthetic bone graft material by using a recombinant protein abbreviated RCPhC1. The development provides a versatile source material to manufacture synthetic bone grafts via flexible engineering. Using preclinical studies in canine and rodent bone defect models, the team showed improved efficiency of the bone grafts to regenerate bone tissue with structural maturity. In clinical orthopedics, the loss of tissue volume and function is a hallmark of injury, chronic inflammation, and metabolic and genetic disease. While bone tissue can actively regenerate via proliferation and osteogenic differentiation of mesenchymal stromal or stem cells, large bone defects require surgical interventions to repair and rebuild bones with bone graft materials.
Globally, orthopedic surgeons perform approximately 2.2 million bone graft procedures annually in an exceedingly costly global market. Human bone tissue is composed of organic extracellular matrix, crystallized calcium and phosphorous minerals that form hydroxyapatite. Bone graft materials can mimic the structure and biochemical composition of bone tissue. Orthopedic surgeons and researchers have used autologous bone grafts (cells and tissue obtained from the same individual) to repair bone defects due to mineral and immunological concerns, although complications at the sites of surgery can lead to alternative grafting methods such as allografts (cells and tissue obtained from a different individual). The newer development of biomimetic, synthetic biomaterials for bone tissue engineering addresses an urgent need in the healthcare industry to develop new graft materials without using human or animal tissue.
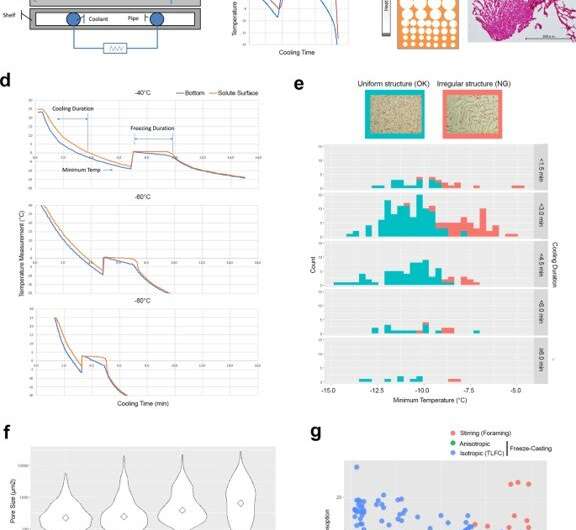
Engineering control factors to develop isotropic ice-templated structures by thin-layer freeze casting (TLFC). (a) Diagram of freeze casting apparatus. (b) Typical temperature measurement of the bottom of the cooling cup (blue) and the solute surface (red) during the freeze casting process. There was a significant discrepancy between the cooling cup and the solute surface during the freezing period. (c) Diagram of anisotropic ice-templated structure and the actual RCPhC1 microstructure containing the small pore zone. Scale bar equals 500 μm. d TLFC reduced the temperature discrepancy between the cooling cup (blue) and the solute surface (red) during the freezing period. (e) The effect of minimum temperature and cooling duration on the isotropic internal pore structure. The uniform pore structure (blue) developed when the minimum temperature was below −10 °C but was not affected by the duration of cooling. The irregular internal pore structure (red) more frequently developed when the minimum temperature was above −10 °C. Scale bar equals 500 μm. Green area shows the counts of uniformly freeze-dried cakes. Red area shows the counts of irregularly freeze-dried cakes. (f) The effect of the block temperature on the internal pore size. Diamonds show median values. The lower block temperatures increased the number of small pores. Scale bar equals 500 μm. (g) The internal structure developed by the stirring (red circles), anisotropic (green circles) and isotropic (blue circles) freeze casting methods was evaluated by water absorption and acid decomposition analyses. RCPhC1 sponges were tentatively dehydrothermal crosslinked at 130 °C for 7 h. Blue lines in panels b and d indicate the temperature record from the thermocouple at the bottom of the solutions. Red lines indicate that at the surface of the solution. Credit: Nature Communications Materials, doi: 10.1038/s43246-020-00089-9
Engineering the recombinant polypeptide and the graft material
The team used type I collagen, abundantly expressed in connective tissues and interstitial membranes as a major organic component of bone tissue. The protein sequence of type I collagen plays an important role in establishing the mechanical strength of bone. The team first cloned the complimentary DNA (cDNA) – a DNA copy of a messenger RNA (mRNA) molecule that encodes the polypeptide (protein sequence) RCPhC1, into an expression vector. To accomplish this, they used a methylotrophic yeast species known as Pichia pastoris to transfer the sequence and generate master and working cell banks. The team confirmed the amino acid composition of the synthetic polypeptide and extensively characterized the product.
They then engineered the internal structure of the graft material to meet specific requirements of the target tissue. To generate a uniform pore structure, therefore, Fushimi et al. designed a thin-layer freeze casting (TLFC) protocol. The versatile freeze approach generated a large number of pores with thin walls to form an isotropic RCPhC1 scaffold with various internal structures.
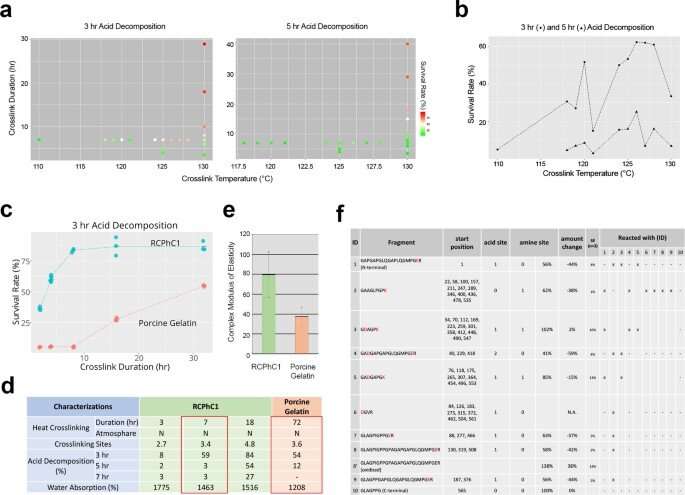
Engineering factors affecting dehydrothermal crosslinking of RCPhC1 to control biodegradation rate and mechanical strength. (a) The effect of crosslinking temperature and crosslinking duration on biodegradation rate evaluated by in vitro acid decomposition analysis. (b) Crosslinking temperature did not linearly influence the acid decomposition survival rate. Considering the downstream sterilization, the crosslinking temperature was set at 130 °C. (c) Crosslinking duration was found to be the predominant control factor, which linearly increased the acid decomposition survival rate of RCPhC1 up to 10 h. Porcine gelatin material responded similarly to the crosslinking duration, albeit requiring a much longer duration. (d) Physical property of dehydrothermal crosslinked RCPhC1. The equivalent physical property of dehydrothermal crosslinked porcine gelatin required 10 times longer crosslinking duration. (e) The mechanical strength of dehydrothermal crosslinked RCPhC1 (130 °C, 7 h) was significantly higher than that of dehydrothermal crosslinked porcine gelatin (130 °C, 72 h). Error bar represents s.d. (f) Dehydrothermal crosslinked RCPhC1 (130 °C, 7 h) was treated with trypsin and subjected to high-performance LC. Co-migration of peptide fragments indicated multiple crosslinking sites. Credit: Nature Communications Materials, doi: 10.1038/s43246-020-00089-9
Dehydrothermal crosslinking profile and optimizing the material for bone regeneration
Fushimi et al. next subjected the material to dehydrothermal crosslinking treatment to test the effect of crosslinking temperature and duration on its composition. After testing the downstream product for microbial contamination, the temperature during the manufacturing process was confirmed to be effective for dry heat sterilization. Additional tests showed how the unique amino acid composition of the recombinant protein contributed to its robust hydrothermal crosslinking efficiency. The team next optimized the recombinant protein material for bone grafting by regulating the concentration of the polypeptide material and its freeze temperature based on the volume of graft-induced bone in a rat calvarial (skull) bone defect model. Four weeks after grafting the material in the animal model, the team used micro-computed tomography (micro CT)-based bone volume estimation. The results indicated an optimal concentration of 7.5 percent RCPhC1, a freezing temperature of -40 to -60 degrees Celsius, and dehydrothermal crosslinking at 130 °C for 7 hours to be best suited for recombinant bone graft material manufacture.
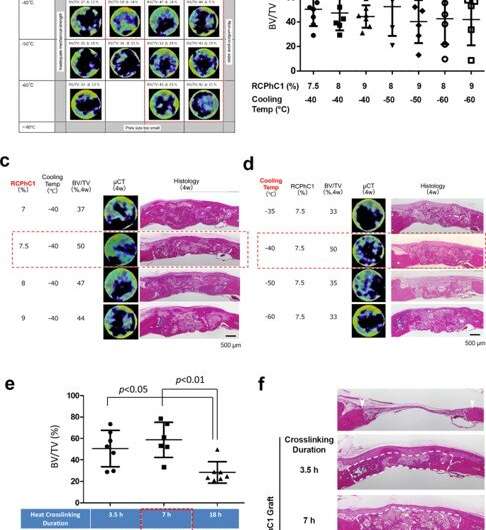
Optimization of engineering control factors. (a) Optimization of combined factors of RCPhC1 solute concentration and TLFC block temperature, with the tentative dehydrothermal crosslinking at 130 °C for 7 h. The optimal range was narrowed down to RCPhC1 concentration between 7.5 and 9% and TLFC block temperature between −40 and −60 °C (red dotted block). (b) BV/TV measurements within the group of the optimal range of RCP1C1 concentration and cooling temperature. There was no statistical difference among groups. (c) The effect of RCPhC1 solute concentration on bone regeneration. Scale bar equals 500 μm. (d) The effect of TLFC block temperature on bone regeneration. Scale bar equals 500 μm. (e) The effect of dehydrothermal crosslinking duration on bone formation. (n = 7; Tukey’s multiple comparison test) (f). The effect of dehydrothermal crosslinking duration on the maintenance of wound healing space in the rat calvarial bone defect. White arrowheads show defect edges. White dotted lines circle newly formed tissue. Scale bar equals 500 μm. Horizontal lines in b, and e represents mean ± s.d. Credit: Nature Communications Materials, doi: 10.1038/s43246-020-00089-9
Robust regeneration of vital bone tissue in preclinical models with graft materials
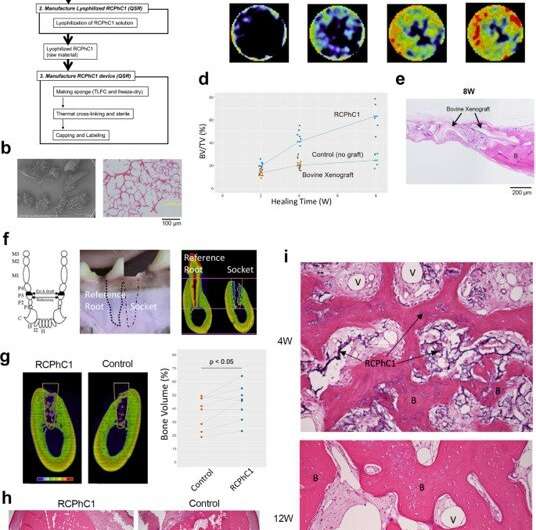
Based on the experimentally verified optimal conditions, Fushimi et al. manufactured the RCPhC1 bone grafts with porous granules. Using the rat calvarial bone defect model, they showed how the bone graft robustly induced bone regeneration within the internal pore structure, while gradually degrading in vivo, to indicate biocompatibility and effective biointegration. They compared this outcome with a commercially available bovine (cow) decellularized cancellous bone xenograft and did not observe significantly greater bone regeneration. The team then tested the bone graft material in a canine preclinical model of tooth extraction to understand wound healing in the tooth socket, where the extraction socket treated with the bone graft showed improved bone formation at 12 weeks. By this time, as expected, the bone graft was largely replaced by bone tissue.
Preclinical efficacy test of the RCPhC1 material optimized for bone graft in rodent and canine models. (a) Flow chart of the RCPhC1 bone graft manufacturing process. Scale bar equals 100 μm. (b) RCPhC1 bone graft was prepared as porous granules of size 300–1200 µm for better handling. Scale bar equals 100 μm. (c) Time course of bone regeneration induced by RCPhC1 bone graft in the rat calvarial defect model. Histological analysis (top row) revealed newly formed bone tissue (B) within RCPhC1 graft material, which was resorbed over time. A time course series of in vivo microCT (bottom row) depicted the progressive increase in bone tissue. (d) Time course of BV/TV measurement of rat calvarial bone defect implanted with RCPhC1 bone graft, bovine decellularized cancellous bone xenograft and no graft control. (e) Histological evaluation of bovine xenograft implanted in rat calvarial bone defect. The physical structure of bovine xenograft was similar to that of RCPhC1 bone graft. At the periphery of the defect, bovine xenograft was seen fused to the newly formed bone (B). In the center of the defect, bovine xenograft granules were surrounded by fibrous tissue. Scale bar equals 200 μm. (f) Canine tooth extraction model. The canine third premolar (P3) has nearly identical mesial and distal roots. The third premolar was hemi-sectioned and only the distal root was extracted. One side of the post-extraction bony socket was filled with RCPhC1 while the other side was left to heal without any further treatment. The remaining medial root was endodontically treated and served as a reference. Scale bar equals 4 mm. (g) MicroCT cross-section depicting bone regeneration in the extraction socket (white line) after 12 weeks (12 W) of healing. The bone volume within the socket was significantly larger in the RCPhC1 bone graft implanted socket than the untreated control socket (n = 8; two-sided paired t-test). Scale bar equals 4 mm. (h) Histological cross section of the extraction socket (white line) at 12 W. The socket that received RCPhC1 bone graft exhibited larger bone regeneration. Dotted white lines show socket interfaces. Scale bar equals 1 mm. (i) Histological evaluation of RCPhC1 bone graft (I) associated bone regeneration. Abundant vascular formation (II) in the bone marrow tissue was noted of regenerating bone (III) after 4 weeks (4 W) of healing. RCPhC1 bone graft was largely resorbed after 12 W of healing. The regenerated bone contained vital osteocytes (IV). Credit: Nature Communications Materials, doi: 10.1038/s43246-020-00089-9
Outlook of synthetic scaffolds in bone tissue engineering
In this way, Hideo Fushimi and colleagues optimized a simple, but critical engineering process to regulate the solute concentration of a recombinant polypeptide of human type I collagen alpha I chain protein with targeted amino acid substitutions, abbreviated as RCPhC1. The team first implanted the construct in a rat calvarial bone defect model to understand the optimal engineering factors to manufacture the bone graft. They designed the bone graft material to support the migration of mesenchymal stem cells (MSCs) toward the defect area and provided a stimulating microenvironment for osteogenic differentiation. The bone graft material alone showed an ideal healing pattern in the absence of growth factors and stem cells to regenerate bone. The material can be used to generate tissue-specific medical devices and graft scaffolds with significant manufacturing versatility in bone tissue engineering.
More information: 1. Fushimi H. et al. Recombinant collagen polypeptide as a versatile bone graft biomaterial, Nature Communications Materials, doi.org/10.1038/s43246-020-00089-9
2. Jacobs P. P. et al. Engineering complex-type N-glycosylation in Pichia pastoris using GlycoSwitch technology, Nature Protocols, doi.org/10.1038/nprot.2008.213
3. Schofer M.D. et al. Electrospun PLLA nanofiber scaffolds and their use in combination with BMP-2 for reconstruction of bone defects, PLOS ONE doi.org/10.1371/journal.pone.0025462
Journal information: Nature Communications , Nature Protocols , PLoS ONE
© 2020 Science X Network