November 20, 2020 feature
Nanobubble-controlled nanofluidic transport
Nanofluidic platforms can offer tunable material transport for biosensing, chemical detection and filtration. Research in the past had achieved elective and controlled ion transport based on electrical, optical and chemical gating methods of complex nanostructures. In a new report now published in Science Advances, Jake Rabinowitz and a team of researchers in electrical engineering, biological sciences and biomedical engineering at the Columbia University, New York, U.S., mechanically controlled nanofluidic transport using nanobubbles. They mechanically generated the nanobubbles made stable via surface pinning and verified them using cryogenic transmission electron microscopy techniques. The findings are relevant for nanofluidic device engineering and nanopipette-based applications.
Investigating the stability of nanobubbles
In this work, Rabinowitz et al. studied how nanobubbles controlled nanofluidic transport by generating metastable nanobubbles in nanopipette channels. Surface-pinned nanobubbles reside at liquid-solid interfaces and can defy physical and thermodynamic predictions of instantaneous dissolution. Researchers have credited the long lifetimes of nanobubbles to a series of effects, including liquid oversaturation with gas and gas accumulation at three-phase interfaces; an insulating oxide, conductive carbon and liquid electrolyte interface. A common feature of these mechanisms is the reduction of the gas-phase concentration gradient between the nanobubble surface and the bulk gas-saturated solution. Surface-pinned nanobubbles present a variety of applications to control (rectify or enhance) ion transport in nanofluidic channels while driving selective mass transport. In broader applications, nanobubbles are suited for water treatment, targeted imaging and drug delivery.
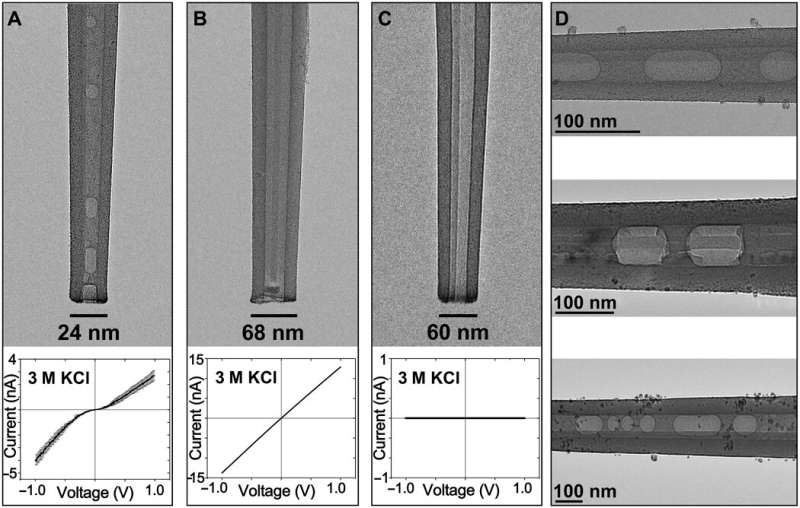
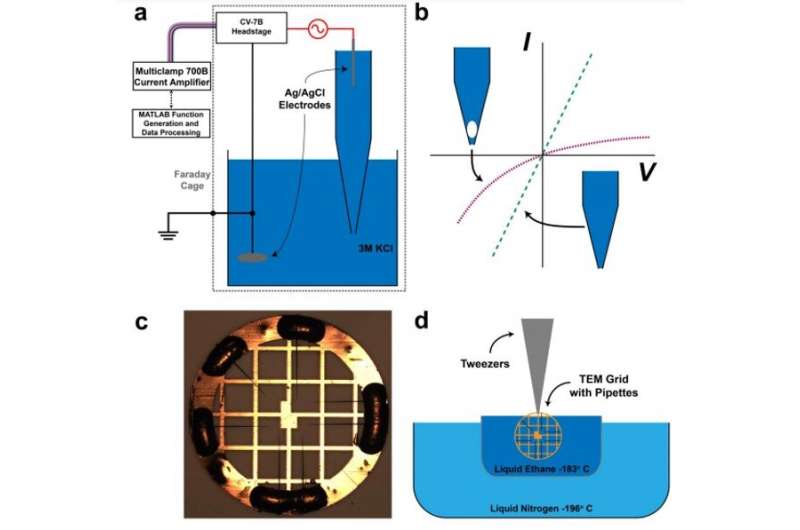
During the experiments, Rabinowitz et al. generated metastable nanobubbles in nanopipette channels by diverting electrolyte flows through interfacial electrolyte films. They confirmed the presence of nanobubbles inside nanopipettes using cryo-electron microscopy (cryo-EM) with transmission electron microscopy. The team monitored the nanobubble-plugged nanopipettes during long-term studies to verify their metastability, and confirmed the outcome using a numerical model.
Detecting nanobubbles with cryo-EM and electronic characterization
Rabinowitz et al. first filled nanopipettes with electrolytes, while holding the tips exposed to air. By removing and re-immersing these pipettes into the electrolyte, they allowed hydrostatic pressure to drive additional electrolytes into the tip while surface tension maintained air voids. The mechanical competition between the hydrostatic pressure and surface tension created nanobubbles in varying sizes, to modify nanobubble configurations within a single nanopipette.
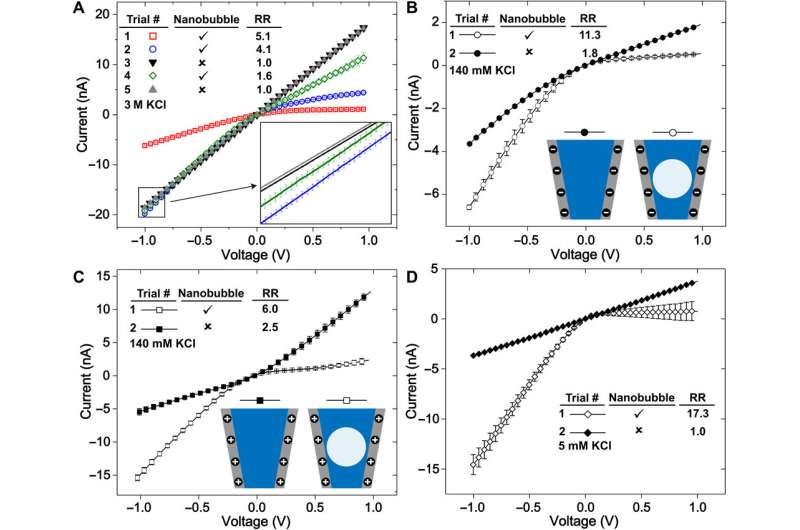
The researchers first measured the ionic currents using a set of uniformly prepared nanopipettes filled with a neutral buffer, where ionic conditions of the surrounding electrolyte determined the nanochannel's current-voltage response. They confirmed the metastability of nanobubbles due to the reproducibility of rectified ionic current measurements, across consecutive voltage sweeps and confirmed the nanobubble occupancy inside nanopipettes using cryo-EM. The team analyzed several electronic measurements prepared for diverse nanobubble configurations to understand how their size influenced nanofluidic transport.
Nanofluidic transport and nanobubble-enhanced ion conductance
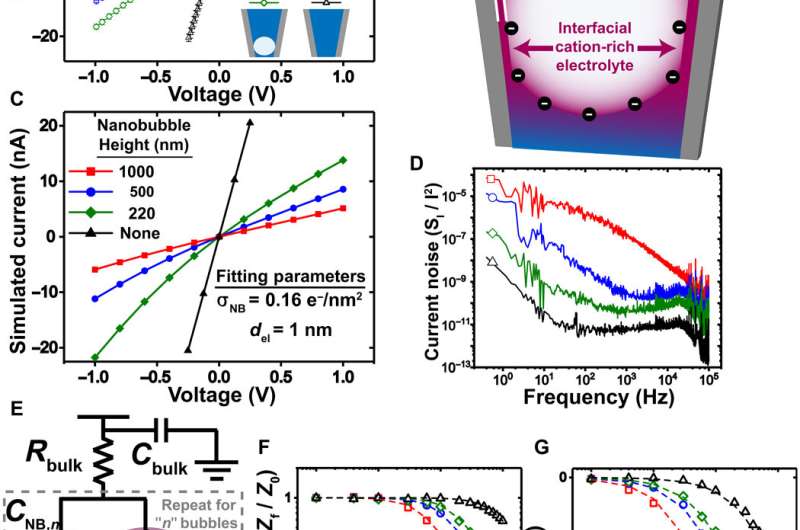
Size dependent changes of nanobubbles could control the nanopipette's fluidic response and modified the nanofluidic transport behavior. The team used ion transport simulations to support the nanofluidic model and replicated the experimental trends by simulating current-voltage responses and impedance simulations to understand the experimental system. The team investigated the pH dependence of nanobubbles, where reduced hydroxide conditions (pH 2) on confined bubbles resulted in a negative charge, while increased hydroxide conditions (pH 12) increased their charge density.
Rabinowitz et al. credited the nanobubble-induced current enhancement to nonlinear electroosmotic flows driven by ion concentration enrichment. For example, intrinsic nanopipette rectification (alternating current-to-direct current power conversion) in the presence of 140 mM potassium chloride (KCl) electrolyte, allowed them to substantiate nanobubbles as the source of conductance enhancement. With further dilution, a nanobubble in 5 mM KCl produced even stronger conductance enhancement and rectification. The team compared the concentration dependence of nanobubble conductance enhancement to observe surface-to-bulk conductance ratios, comparable to those observed in surface charge-governed transport through a nanopore.
Nanobubble metastability model
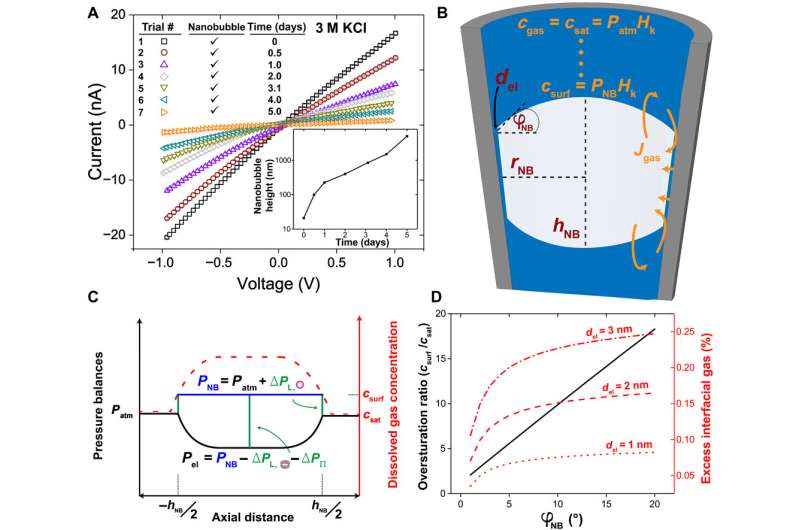
The team then used reproducible and geometry-dependent measurements, to show the stability of nanobubbles over a period of minutes, unperturbed by electric fields. By monitoring long-term bubble-plugged nanopipettes, they noted slow nanobubble growth, where a nanopipette containing 3M KCl showed a rectification ratio of 1.3 and an average resistance of 54 megaohms. Rabinowitz explained the steady nanobubble growth in gas oversaturated liquid using a dynamic equilibrium model for nanobubble-electrolyte gas exchange and estimated the dissolved gas concentration at the nanopipette wall using finite element modeling and gas law relations.
Outlook
In this way, Jake Rabinowitz and colleagues characterized ion transport through nanobubble-plugged nanopipettes and observed nanobubble metastability under these conditions. The team demonstrated composite nanochannels with tunable ionic currents, atomically thin electrolyte films and effective apertures comparable to biological ion channels. The team showed the ability to improve nanochannel conductivity in the forward rectification direction and credited the observations to nonlinear electrokinetic phenomena. They developed a mechanical technique in this study to generate nanobubbles inside nanopipettes and fabricate these transport systems. The transport effects detailed in this work are relevant to applications that rely on ionic currents through nanopipettes, including patch clamp electrophysiology and scanning ion conductance microscopy. In addition to that, the phenomenon of long-term nanobubble growth without an external source of gas oversaturation presents a new system that can provide insight into three-phase interface dynamics.
More information: Jake Rabinowitz et al. Nanobubble-controlled nanofluidic transport, Science Advances (2020). DOI: 10.1126/sciadv.abd0126
Joost H. Weijs et al. Why Surface Nanobubbles Live for Hours, Physical Review Letters (2013). DOI: 10.1103/PhysRevLett.110.054501
Matthew R. Powell et al. Electric-field-induced wetting and dewetting in single hydrophobic nanopores, Nature Nanotechnology (2011). DOI: 10.1038/nnano.2011.189
Journal information: Science Advances , Physical Review Letters , Nature Nanotechnology
© 2020 Science X Network