Credit: University of Oklahoma
A collaborative research team from the University of Oklahoma, the Memorial Sloan Kettering Cancer Center and Merck & Co. published an opinion article in the journal, Nature Chemical Biology, that addresses the gap in the discovery of new antibiotics.
"The continuing emergence of antibiotic-resistant bacteria, and our inability to develop new antibiotics to combat them, represent two of the most wicked healthcare problems we face," said OU vice president for research and partnerships Tomás Díaz de la Rubia. "OU's team is working hard to develop solutions to these major challenges, and their opinion article helps bring visibility and attention to these issues at a time when it's needed most."
The research team is composed of OU researchers Helen Zgurskaya, Valentin Rybenkov and Adam Duerfeldt, all in the Department of Chemistry and Biochemistry, College of Arts and Sciences, with researchers from the Memorial Sloan Kettering Cancer Center, led by Derek Tan, and corporate researchers from Merck & Co.
"The rapid spread of antibiotic-resistant bacteria in clinics challenges our modern medicine and the traditional approaches to antibiotic discovery fail to generate new drugs needed for treatment of antibiotic resistant infections," Zgurskaya said. "The current COVID-19 pandemic further magnifies this problem because patients in intensive care units are particularly vulnerable to such infections ... (our) team is working on developing new tools to guide the discovery and optimization of new antibacterial agents."
Zgurskaya adds that the increasing frequency of antibiotic resistance has created a significant health care challenge and will progressively worsen without innovative solutions.
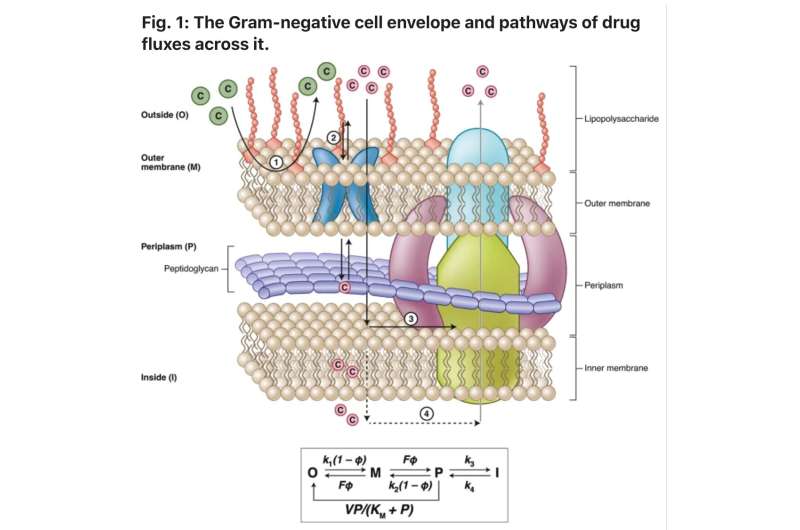
"In particular, Gram-negative pathogens present both biological and chemical challenges that hinder the discovery of new antibacterial drugs," Zgurskaya said. "As a result of these challenges, intensive screening campaigns have led to few successes, highlighting the need for new approaches to identify regions of chemical space that are specifically relevant to antibacterial drug discovery."
In the article, the research team provides an overview of emerging insights into this problem and outline a general approach for researchers and scientists to address it.
"The overall goal is to develop robust cheminformatic tools to predict Gram-negative permeation and efflux, which can then be used to guide medicinal chemistry campaigns and the design of antibacterial discovery libraries," Zgurskaya said.
More information: Shibin Zhao et al, Defining new chemical space for drug penetration into Gram-negative bacteria, Nature Chemical Biology (2020). DOI: 10.1038/s41589-020-00674-6
Journal information: Nature Chemical Biology
Provided by University of Oklahoma