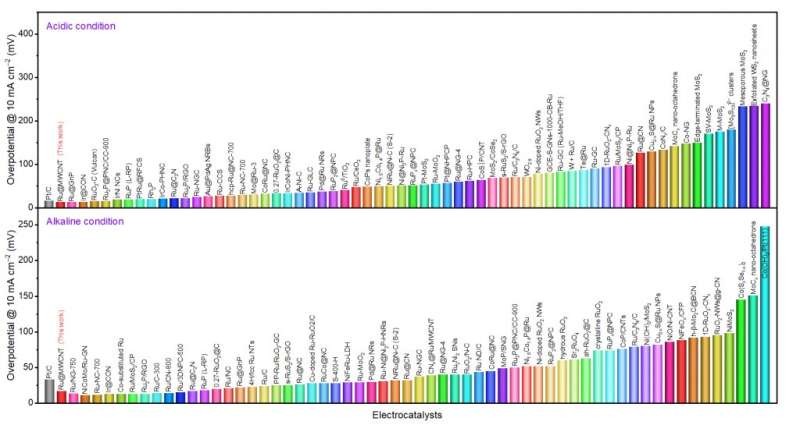
Figure 1. Comparison of the overpotentials at 10 mA cm−2 with recently reported HER catalysts in both acidic and alkaline conditions. Credit: Professor Jong-Beom Baek, UNIST
Researchers throughout the world are working actively to accelerate the development of new catalysts that can greatly cut the cost of hydrogen production. A number of breakthrough catalysts have been reported, yet their expected performance are often unknown before implementation, and thus further research is required for practical use. A recent study, affiliated UNIST, has introduced a novel highly-efficient catalyst for hydrogen generation and its expected catalytic performance has been also demonstrated.
Professor Jong-Beom Baek and his research team in the School of Energy and Chemical Engineering at UNIST have successfully developed a new water-splitting hydrogen catalyst, which consists of ruthenium (Ru) nanoparticles uniformly distributed and anchored on the surface of multiwalled carbon nanotubes (MWCNTs), or (Ru@MWCNT). The research team also evaluated the catalytic performance of the Ru@MWCNT. The results indicated that the Ru@MWCNT catalyst is superior in many ways to the commercial Pt/C catalysts. The new catalysts are simple to synthesize and can be mass-produced, according to the research team.
"In addition to introducing highly-efficient and stable catalysts that surpass the characteristics of existing materials, this study aims at evaluating the catalytic performance of catalyst electrodes, which is an essential part of commercialization," says Professor Baek.
Hydrogen is the most abundant element, which accounts for 75% of the universe, and has been regarded as an efficient and environment friendly power source for the future. Currently, the majority of hydrogen is produced from fossil fuels, such as natural gas and this often release carbon dioxide (CO2) emissions in the process. As an alternative, the process of using electricity to split water into hydrogen and oxygen has been suggested, but this requires the usage of expensive catalysts, such as platinum.
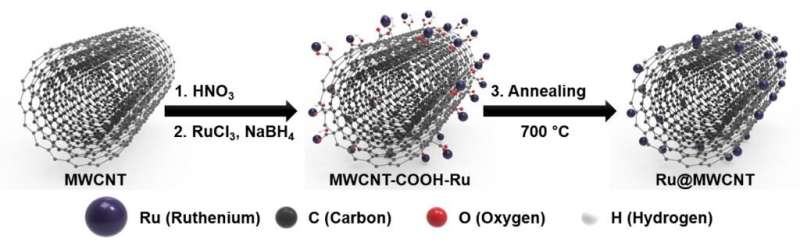
Figure 2. Schematic illustration of the process steps for forming Ru@MWCNT catalyst. Credit: Professor Jong-Beom Baek, UNIST
Hence, Professor Baek's team has been steadily developing catalysts that are not only superior in performance to traditional platinum catalysts, but have lower production costs. The Ru@MWCNT catalyst exhibits superior electrochemical properties over the previously announced metal-organic catalysts. The catalyst demonstrates excellent HER performance with low overpotentials (See Figure 1), outstanding durability and high turnover frequencies in both acidic and alkaline conditions.
The Ru@MWCNT catalysts take the structure, in which ruthenium (Ru) nanoparticles are uniformly distributed and anchored on the surface of multiwalled carbon nanotubes (MWCNTs). Thanks to the smaller particle size distribution and particle uniformity, it displays excellent HER performance and for this, a manufacturing process has been also developed.
"The existing method of combining Ru and CNTs, there is a tendency for Ru particles stick together and by steadily increasing the size of the agglomerate during the heat treatment," says Do Hyung Kweon (Combined M.S/Ph.D. of Energy and Chemical Engineering, UNIST), the first author of the study. "We suppress this particle agglomeration via the introduction of 'Ru salt' and '-COOH' and this enabled the uniform distribution of Ru nanoparticles on the surface of the MWCNT."
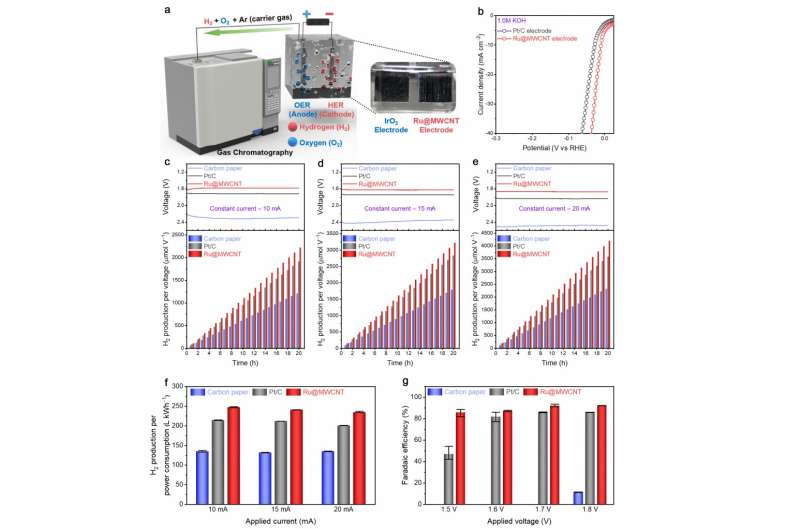
Figure 3. HER performance evaluation in actual water splitting. Credit: Professor Jong-Beom Baek, UNIST
In order to accurately determine the performance of the new catalyst, Professor Baek has conducted the HER performance evaluation in the actual water-splitting system construction and analysis, in addition to the existing overpotential measurement. Their results show that Ru@MWCNT produces 15.4% more hydrogen per power consumption than commercial Pt/C and Faradaic efficiency (92.28%) is higher than Pt/C (85.97%).
"Previous studies on hydrogen catalysts focus on the evaluation of the catalytic performance itself, and they were inadequate to deal with the actual water-splitting system construction and analysis," says Professor Baek. "This study is significant as it can predict the actual HER applicability."
The findings of this research have been published in Nature Communications.
More information: Do Hyung Kweon et al. Ruthenium anchored on carbon nanotube electrocatalyst for hydrogen production with enhanced Faradaic efficiency, Nature Communications (2020). DOI: 10.1038/s41467-020-15069-3
Journal information: Nature Communications