A new strategy for the synthesis of crystalline graphitic nanoribbons
New work from a team of scientists led by Drs. Kuo Li and Haiyan Zheng from the Center for High Pressure Science and Technology Advanced Research (HPSTAR) collaborated with Dr. Jing Ju from Peking University found pressure-induced polymerization of 1,4-diphenylbutadiyne produces crystalline graphitic nanoribbons. Their study provides a new strategy to synthesize crystalline bulk graphene nanoribbons with atom-scale ordering and controlled width. The result is published recently in Journal of the American Chemical Society.
Graphitic nanoribbons (GNRs) are strips of graphene, which has a non-zero bandgap and shows great potential application in the area of nano-scale electronic and optoelectronic devices. The band gap is closed related to its width, backbone and edge structures as well as atomic level substitutions. Thus, the synthesis of atomically precise GNRs is very critical. The 'bottom-up' method including the surface-assisted and solution-mediated synthesis method is an attractive protocol to construct the GNRs with desired structure. However, these two methods are not suitable for synthesizing bulk crystalline GNRs.
One promising approach to obtain crystalline products is solid-state topochemical polymerization, which can be induced in a constrained crystallized environment under external physical stimuli (light, heat, pressure, etc.). Unfortunately, the reaction types of the SSTP are limited to a few types, such as 1,4-addition, [2+2] cycloaddition and azide-alkyne cycloaddition. The most widely used Diels-Alder (DA) and Dehydro-Diels-Alder (DDA) reaction for building a new six-membered carbocycle in solution are scarcely seen in solid-state reaction, because achieving the proper orientation and distance between a diene and a dienophile is extremely challenging.
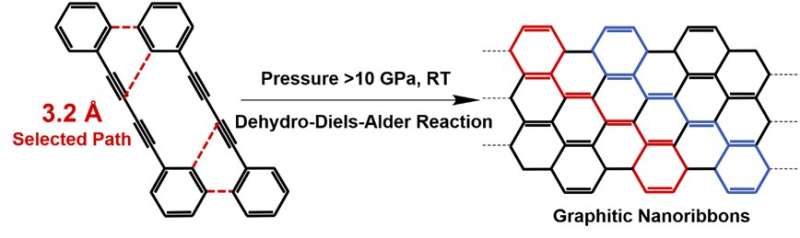
Pressure-induced polymerization (PIP) has shown its unique advantages in the synthesis of various novel crystalline materials, because pressure is the most effective way to regulate the crystal structure and compressing the intermolecular distance of reactant. By using in situ Raman and IR spectroscopy, the authors found that the PIP of 1,4-diphenylbutadiyne (DPB) starts via an unexpected DDA reaction with phenyl as dienophile instead of 1,4-addition reaction between diynes. By using multiple cutting-edge techniques, the authors confirmed that the product are crystalline armchair graphitic nanoribbons. It has a graphene nanoribbon structure with sp3-carbons at the edge. We can expect that the sp3 carbon can convert to sp2-carbons by losing hydrogen and a well-defined GNR structure with a clear armchair edge and width of 1 nm will be produced.
Furthermore, the researchers also performed in situ high-pressure neutron diffraction to explore the crystal structure of DPB at the reaction threshold pressure (10 GPa) and the critical distance of this DDA reaction was determined as 3.2 Å. Based on several quantitative distances of the different reactive positions before reaction, they proposed that the PIP is dominated by the distance of reactive positions, which is different from the solution reaction dominated by the active of functional groups.
More information: Peijie Zhang et al, Distance-Selected Topochemical Dehydro-Diels–Alder Reaction of 1,4-Diphenylbutadiyne toward Crystalline Graphitic Nanoribbons, Journal of the American Chemical Society (2020). DOI: 10.1021/jacs.0c08274
Journal information: Journal of the American Chemical Society
Provided by Center for High Pressure Science & Technology Advanced Research