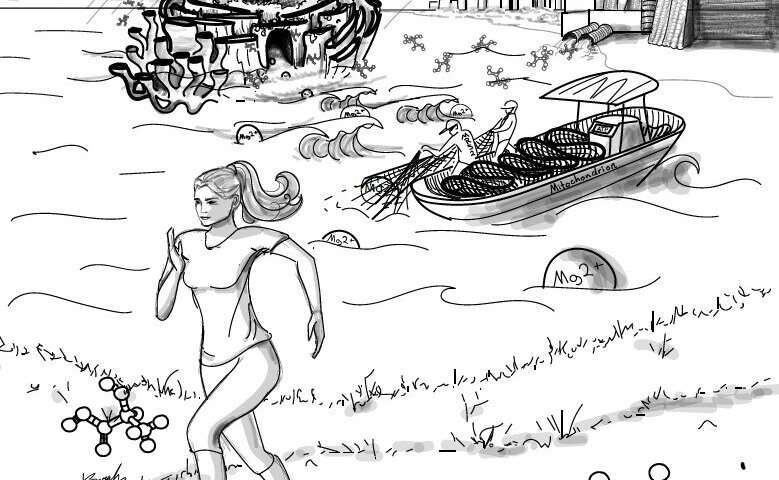
The most abundant biological divalent cations, Ca2+ and Mg2+, antagonistically regulate divergent metabolic pathways with several orders of magnitude affinity preference. In this issue, Daw, Ramachandran, et al. identified lactate as the endogenous ligand to generate spatio-temporal Mg2+ dynamics that decodes the signal transduction into mitochondrial energetics. The cover image depicts the strenuous exercise-induced, paracrine-derived lactate surge into hepatocytes which elicits endoplasmic reticulum Mg2+ release as waves that are tunneled into mitochondria through its Mg2+ transporter, Mrs2, in a thermosensing fashion. Credit: Sarah Bussey.
Researchers from The University of Texas Health Science Center at San Antonio (UT Health San Antonio) have solved the 100-year-old mystery of what activates magnesium ions in the cell. The discovery is expected to be a springboard for future development of novel drugs to treat cardiovascular disease, metabolic disorders such as diabetes, and other diseases.
Reporting Thursday (Oct. 8) in Cell, scientists in the Joe R. and Teresa Lozano Long School of Medicine at UT Health San Antonio said the magnesium activator is a metabolite called lactate, which is elevated in the blood during intense exercise and in many diseases, including heart disease, diabetes, sepsis and cancer.
"Lactate is a signal that—like a light switch—turns on magnesium ions," said lead author Madesh Muniswamy, Ph.D., professor of cardiology in the Long School of Medicine. "On lactate's signal, the ions rush out of cellular storehouses called the endoplasmic reticulum."
The team made a second discovery: A protein called Mrs2 transports the released magnesium ions into cell powerhouses known as mitochondria. These power plants generate ATP, which is the energy currency fueling all the processes in the body.
"We believe this loop is essential for health," said study coauthor W. Brian Reeves, MD, chairman of the Department of Medicine at UT Health San Antonio. "If there is a problem with magnesium routing, impairments ensue, such as the diminished mitochondrial function and poor energy production observed in Type 2 diabetes or severe infections."
IP3, the activator for calcium ions, was discovered in 1984. Since that time, the calcium field has grown in monumental fashion, whereas magnesium continued to be a riddle, said coauthor Karthik Ramachandran, Ph.D., postdoctoral fellow in the Muniswamy laboratory.
Coauthor Travis Madaris, a graduate student on the research team, said, "As a student in the lab, this discovery is exciting because it lays out a pathway for multiple publications while I'm in this lab, and most importantly, it can lead to many future discoveries to improve human health."
Madaris is supported by a predoctoral research training fellowship awarded by the National Institutes of Health.
Summing up the discovery, Dr. Muniswamy said: "Magnesium is essential for life. It's in our blood. It's been implicated in and used as a treatment for a variety of diseases, including migraines, cardiovascular diseases, diabetes and preeclampsia. But to take the next step forward, we needed to understand the dynamics of magnesium in our bodies. With this finding, we believe we have laid out one of the pillars of support that the scientific world needed."
More information: Lactate Elicits ER-Mitochondrial Mg2+ Dynamics to Integrate Cellular Metabolism, Cell (2020). DOI: 10.1016/j.cell.2020.08.049
Journal information: Cell