The researchers showed that their yeast- and silkworm-derived enzyme far outperformed commercial sources of the enzyme made in standard expression systems. Credit: KAUST; Heno Hwang
A new way of producing an enzyme called fucosyltransferase VI (FTVI) in the lab could help enhance the therapeutic potential of cord blood transplants.
Cord blood is currently used to treat more than 80 life-threatening conditions, ranging from cancer and immune deficiency to metabolic and genetic disorders. The therapy is predicated on the idea that stem cells in the cord blood will traffic to the bone marrow, where they can help rebuild a healthy blood and immune system that has been damaged by disease. But cord blood stem cells are not naturally adept at this process—which is why several drugmakers have turned to FTVI as a way of enhancing the cells' homing ability.
FTVI is an enzyme involved in tagging cells with sugar molecules in a way that alters migration patterns in the body. In clinical trials, cord blood stem cells treated with FTVI showed enhanced engraftment following infusion into cancer patients. Yet most commercial sources of FTVI available today have only limited enzymatic activity. Plus, they tend to be made using various expression systems that either produce enzymes with low activity or are costly and generate low yields.
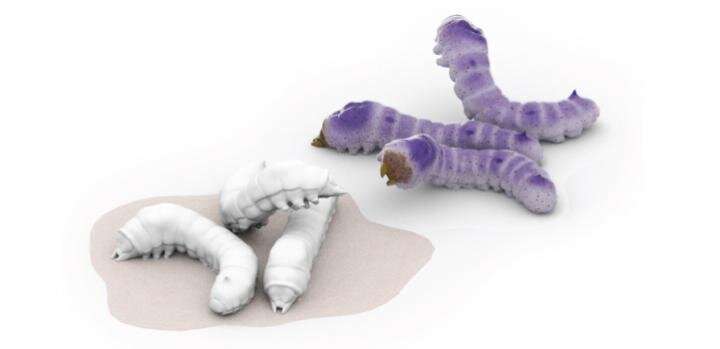
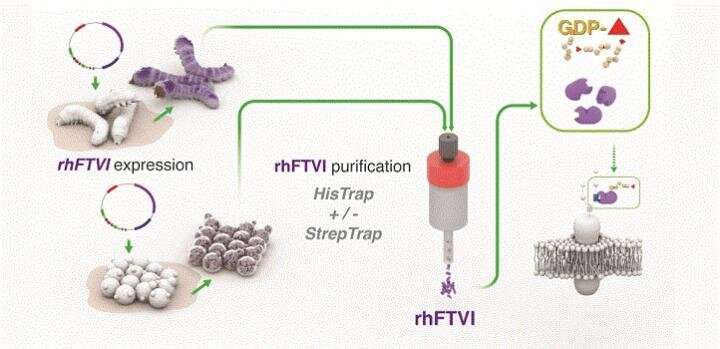
Seeking a better manufacturing platform, a team led by Jasmeen Merzaban at KAUST engineered yeast cells and silkworm larvae to express the human version of FTVI. Working with collaborators in Japan, KAUST researchers from several teams came together to devise a purification scheme for obtaining the enzyme at high yields; they then tested how efficiently the end-product could alter human stem cells.
Two expression systems, a silkworm and a yeast, were shown to produce significant amounts of enzymes that are better than currently available commercial enzymes that assist with cord blood transplants. Credit: Al-Ammodi et al.,KAUST, Heno Hwang
The researchers showed that their yeast- and silkworm-derived FTVI far outperformed commercial sources of the enzyme made in standard expression systems. "Now, these enzymes can be usedex vivoon stem cells to enhance their migration toward the bone marrow during a transplant," Merzaban says.
Alternatively, researchers could take advantage of the new yeast- and silkworm-produced FTVI for drug screening efforts. First author of the study, Asma Al-Amoodi, points out that many metastatic cancers exhibit enhanced activity of FTVI and similar enzymes. "We could envision using such enzymes to screen for small molecule inhibitors that block metastasis," she says.
More information: Asma S. Al-Amoodi et al. Using Eukaryotic Expression Systems to Generate Human α1,3-Fucosyltransferases That Effectively Create Selectin-Binding Glycans on Stem Cells, Biochemistry (2020). DOI: 10.1021/acs.biochem.0c00523
Journal information: Biochemistry