September 8, 2020 feature
Real-time imaging shows how SARS-CoV-2 attacks human cells
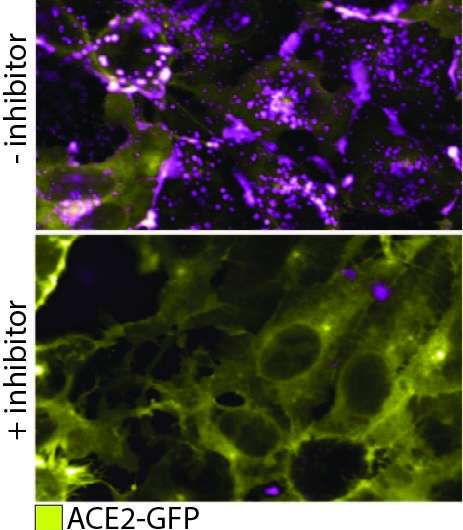
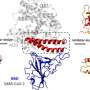
"What we're doing here is actually visualizing binding of the spike to ACE 2 [angiotensin converting enzyme 2]," says Kirill Gorshkov a research scientist at the National Center for Advancing Translational Sciences (NCATS) in Maryland, U.S.
Innocuous though this may sound to the uninitiated, this binding is the first step in a process of viral proliferation that may have led to the worst pandemic in living memory. The "spike" is a protein on the SARS-CoV-2 virus that is widely recognized as the primary weapon of attack for mobilizing its viral DNA into a host cell. The ACE2 receptors are human cell proteins that effectively open the door for this attack. Using bioengineered quantum dots, Gorshkov and Eunkeu Oh at the Naval Research Laboratory (NRL) in Washington, D.C., and their colleagues were able to image the binding and subsequent internalization that takes place when ACE2 and the spike protein interact. "You can actually see that happen in real time," adds Gorshkov, "That's the beauty of this assay and that's why we think it will be important for drug screening."
A virus cannot reproduce without enrolling a host cell, so researchers around the globe have been working to understand how SARS-CoV-2 interacts with and penetrates cells with the aim of blocking this stage and preventing the onset of COVID19. Gorshkov and his colleagues at NCATS were already working on various imaging assays for cancers, viruses and lysosomal storage diseases, "but when coronavirus hit, we quickly had to shift gears," says Gorshkov.
Prior SARS research had highlighted the importance of interactions with ACE2 in human cells for the spread of this kind of virus, and they were already able to tag these receptor proteins with a green fluorescent protein to image their movements. Evidence was also accumulating to pinpoint the specific spike proteins on SARS-CoV-2 that might be locking ACE2 into a stronghold so that the virus can enter the cell. However, information on spike protein interactions has mostly accrued indirectly from biochemical or proximity assays and tests with proteins and parts of proteins taken from the virus—"pseudo-viro-particles." With no fluorescent labeling of these viral proteins, their role in the ACE2 receptor binding and subsequent internalization—endocytosis—continued to play out effectively under cover of darkness to imaging.
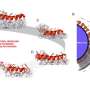
At NRL, researchers were also keen to leverage their expertise with nanoparticles for cellular delivery and biosensing to help efforts in search of anti-COVID19 drugs. Oh began looking into possible ways of applying the protein-nanostructure conjugation techniques she had been working with for over 15 years. With two proteins that share a binding affinity—a quantum dot attached to one and a fluorescing nanoparticle attached to the other—binding between the two proteins will then bring the nanostructures close enough for energy transfer between them.
The resulting fluorescence quenching then allows the researchers to monitor the protein binding. "If you have any inhibitor in the middle to stop the binding, this can be used as an inhibition assay for drug screening, so we use this a lot," explains Oh. Seeing the potential application for screening antibodies against COVID19, Oh and her team led by Mason Wolak presented their ideas to the team at NCATS, and the two institutions set straight to work to develop it further.
Developing a "pseudovirion"
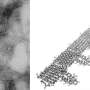
The first step from Oh's side of the collaboration was to develop a "pseudovirion" with the potent parts of SARS-CoV-2 spike proteins (where the receptor binding domain is situated) attached to the quantum dot in such a way that the spike proteins continue to attack and penetrate cells just like an active virus. For this, the orientation of the spike proteins and the shape of the pseudovirion was key, and here, Oh's extensive experience conjugating active proteins to nanostructures paid off. Before moving on to the more expensive cellular delivery tests, they had to test whether their pseudovirion was functioning outside of cells by conjugating fluorescing gold nanoparticles to the ACE2 receptors and monitoring for fluorescence quenching. Oh lists the multiple ratios of protein to quantum dot, quantum dot sizes and surface chemistries they tried before they were finally able to observe fluorescence quenching on protein binding, and were ready to send the pseudovirion to Gorshkov's team "to do cool stuff with the real cell."
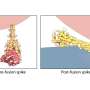
To observe the pseudovirion interacting with ACE2 in a real cell, the quantum dot on the pseudovirion now needed to be engineered to emit at a wavelength that was easy to distinguish from the green fluorescent protein on ACE2, as opposed to optimizing nanoparticle quenching. With the two clear signals, the team at NCATS could track the binding of the two proteins and subsequent endocytosis. Additionally, they could see that the binding and endocytosis was prevented in the presence of two test antibodies. They could even test the endocytosis mechanism, which proceeds by means of a protein named dynamin. When they added Dyngo-4a, which interrupts dynamin, they could see the binding take place but no subsequent endocytosis.
The results also chalk up a success for remote research collaborations, as the teams never actually met. "The type of collaboration we have here is a rare one," says Gorshkov, reflecting how much their progress outpaced previous collaborations where there had been a greater number of physical meetings and coordinated activities. "There was such a drive and such a focus from both groups that it really synergised very well."
The quantum dot pseudovirion is limited to imaging cell penetration by endocytosis, and it remains to be determined whether this mechanism comes into force for all cell types, lung tissue in particular. An alternative SARS-CoV-2 attack mechanism is based on membrane fusion, and imaging this with the quantum-dot pseudovirion would require significant modifications to interact with the cell more like a membrane. However, the fast throughput and direct observations the quantum-dot pseudovirion enables should pose significant advantages in the search for antibodies.
More information: Kirill Gorshkov et al. Quantum-Dot-Conjugated SARS-CoV-2 Spike Pseudo-Virions Enable Tracking of Angiotensin Converting Enzyme 2 Binding and Endocytosis, ACS Nano (2020). DOI: 10.1021/acsnano.0c05975
Journal information: ACS Nano
© 2020 Science X Network