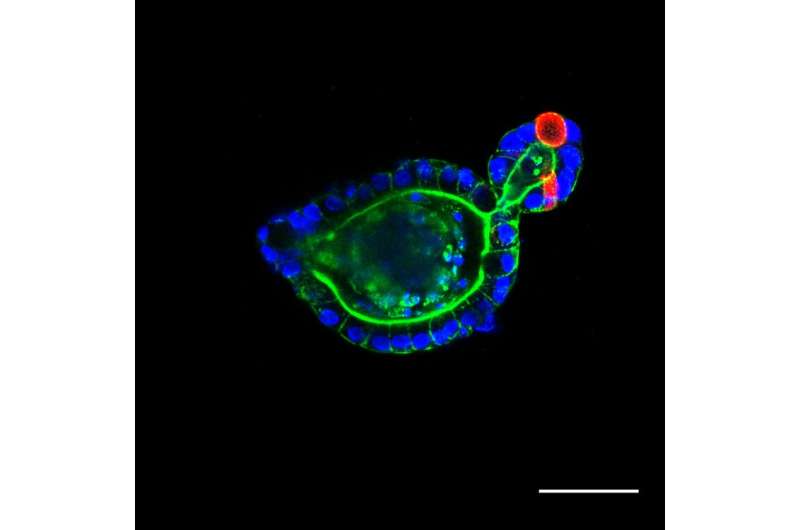
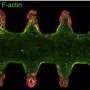
An intestinal organoid that has been passaged from our photodegradable materials. Credit: University of Colorado at Boulder
Max Yavitt, a graduate student in the Department of Chemical and Biological Engineering, is the first author on a new paper in Advanced Materials focusing on organoid development. We asked Yavitt about the research problems the work explores, his time with the Anseth Lab and why this is such an important and growing area of research–particularly for the field of medicine.
Question: How would you describe the work and results of this paper?
Answer: "This work uses photodegradable hydrogels to support the long-term growth of intestinal organoids. Intestinal organoids are cellular constructs that resemble intestinal tissue, but can be easily grown in a lab. These organoids require a supportive scaffold to grow and develop in three dimensions.
Once intestinal organoids grow too large, it becomes difficult to maintain their structure and function. So they must periodically be physically broken into smaller structures. This process is termed "passaging "and is required for long term organoid growth. In this work, we use a scaffold that is photodegradable, meaning that it can be dissolved by the application of UV light. This affords us much greater control over organoid passaging than conventional materials. In this work, we first look at the chemical reaction that enables degradation and optimize it for organoid culture. We identified new conditions that make this reaction occur much faster than previously reported. We then optimize conditions for organoid growth within these scaffolds and use photodegradation as a mechanism to passage organoids. We finally show that organoid function and health is not adversely affected by this passaging mechanism."
Q: What are some possible applications for this work?
A: "Organoids can be thought of as mini-organs and researchers are interested in them because they are a manageable and lab-scale replacement for studying anything from organ function and development, to disease progression and even the effect of drug treatments. This type of research usually involves the use of live animals which is often expensive and time consuming. Instead, researchers can generate thousands of organoids in a lab dish in a matter of days or weeks and then use these cells to answer similar questions relating to organ and tissue behavior. Organoids have been developed for a variety of organ and tissue types including brain, kidney, liver, lung, intestine, heart and many others.
One limitation to using organoids in clinical applications is the materials in which they grow. Organoids are commonly grown in supportive scaffolds composed of a heterogenous mixture of proteins. The exact composition of these materials is often unknown, difficult to determine and widely varied from batch to batch. This creates challenges for standardization and reproducibility.
In response, our work is focused on developing synthetic polymeric materials for organoid development. Polymers are synthesized in a lab, and the exact composition can be rigorously determined and reproduced. In this manner, we can eliminate the uncertainty involved in conventional organoid culture. We hope that these synthetic materials will become the norm for organoid culture, allowing for more standardized, reproducible, and consistent results."
Q: Is this a research topic or area you were interested in before joining the Anseth Lab?
A: "To be honest, I didn't know what organoids were before joining the Anseth Lab. I was generally interested in developing materials that interfaced with cells, but I didn't know what types of biological questions I wanted to answer.
Now that I am in graduate school and in the Anseth Lab, I am extremely grateful that I am able to work with organoids because they are such an interesting and unique cell construct. I think that organoids are an amazing tool that have the ability to revolutionize certain areas of biomedical research, yet there is still so much that we don't know about organoid growth and development. I am so lucky to be able to contribute to this burgeoning branch of research."
Q: Was there a particular aspect of this work that was hard to complete or with?
A: "As with most biological research, actually working with the cells was the most difficult. I had no experience with cell culture coming into graduate school, so I had to learn how to maintain and care for these cells. It took a lot time to get to the point where I could keep them alive and healthy long enough to be able to do all of the necessary experiments. My training is in chemical engineering, so I was much more comfortable with the materials development aspect of this research. However, it has been fun to learn more biology and cell culture techniques."
Q: What research questions are still to be answered in this area? Where will the work go after this paper?
A: "There are still a lot of questions around how cells interact with their environment, and how these interactions influence organoid development. Cells are very responsive to their surroundings, and even have the ability to sense how stiff or soft their environment is. These mechanical signals are known to influence organoid development but have been difficult to study due to a lack of materials that easily enable these investigations.
We believe that our photodegradable materials, which afford precise control over the material's mechanical properties, will allow us to learn a great deal about organoid growth in response to mechanical stimuli. This knowledge will motivate the formation of new materials to optimize organoid growth for clinical applications."
More information: F. Max Yavitt et al. The Effect of Thiol Structure on Allyl Sulfide Photodegradable Hydrogels and their Application as a Degradable Scaffold for Organoid Passaging, Advanced Materials (2020). DOI: 10.1002/adma.201905366
Journal information: Advanced Materials
Provided by University of Colorado at Boulder