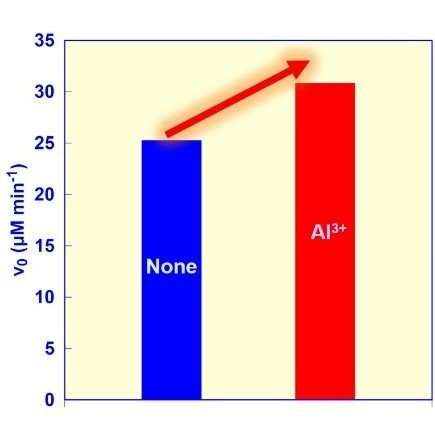
The apparent rates for the ME-catalyzed malate (v0) under the condition of NADPH, pyruvate, sodium bicarbonate and ME containing aluminium ions (red). Credit: Yutaka Amao, Osaka City University
Carbon dioxide (CO2) levels are rising and our planet is heating up. What do we do? What if we used this excess CO2 as a raw material to produce things we need—similar to how plants use it to produce oxygen.
This is one thing artificial photosynthesis has set out to do.
Artificial photosynthesis is a chemical process that mimics the natural process of photosynthesis to convert sunlight, water, and carbon dioxide into useful things like carbohydrates and oxygen. The problem is that current technologies can only produce molecules with 1 carbon atom. These molecules are too weak to be used for the production of more complex materials. Standard experimental conditions have not been stable enough to allow for molecules with bonds of more than one carbon atom to form.
New research at Osaka City University has found that simply adding metal ions like aluminum and iron was enough to allow the production of malic acid, which contains four carbon atoms. The study appeared recently online in the New Journal of Chemistry published by the Royal Society of Chemistry.
"I was surprised that the solution was found in such a common thing as aluminum ions," said lead author Takeyuki Katagiri.
"Our goal is to create groups of molecules with as many as 100 carbon atoms," added supporting author Yutaka Amao. "Then we can finally explore possibilities of using CO2 as a raw material."
More information: Takayuki Katagiri et al. Trivalent metal ions promote the malic enzyme-catalyzed building of carbon–carbon bonds from CO2 and pyruvate, New Journal of Chemistry (2020). DOI: 10.1039/D0NJ03449E
Provided by Osaka City University