Exploring oxidative pathways in nuclear fuel

Powerful atomic-resolution instruments and techniques at Pacific Northwest National Laboratory (PNNL) are revealing new information about the interaction of uranium dioxide (UO2) with water. These new insights will improve the understanding of how spent nuclear fuel will degrade in deep geologic repository environments.
UO2 is the primary form of fuel used in commercial nuclear power reactors. During nuclear fission in a reactor, various radionuclides are created within the fuel. Researchers want to know more about UO2, particularly the dissolution mechanisms that come into play when the ceramic material's surface contacts water. These mechanisms control the release of the majority of the radionuclides, which could have implications for the environment.
Many laboratory instruments today lack the sensitivity, resolution, and radiological controls necessary to effectively explore UO2 surfaces. However, a one-of-a-kind instrumentation suite at PNNL recently enabled a multi-institute research team to take a closer look at surface areas. The team, representing the University of Cambridge, the European Commission's Joint Research Center, and PNNL, uncovered key revelations for nuclear energy.
Geologic disposal and science challenges
Deep geologic repository concepts being proposed around the world are focused on the saturated zone, where the water is reducing—which can eventually lead to a loss of oxygen—and where UO2 is thermodynamically stable. The challenge remains to develop an approach to examine UO2 with sufficient chemical resolution and fidelity to predict how it might behave in these environments.
"We're just now developing the tools we need to answer longstanding questions about nuclear materials," explains PNNL materials scientist Edgar Buck.
New techniques produce new information
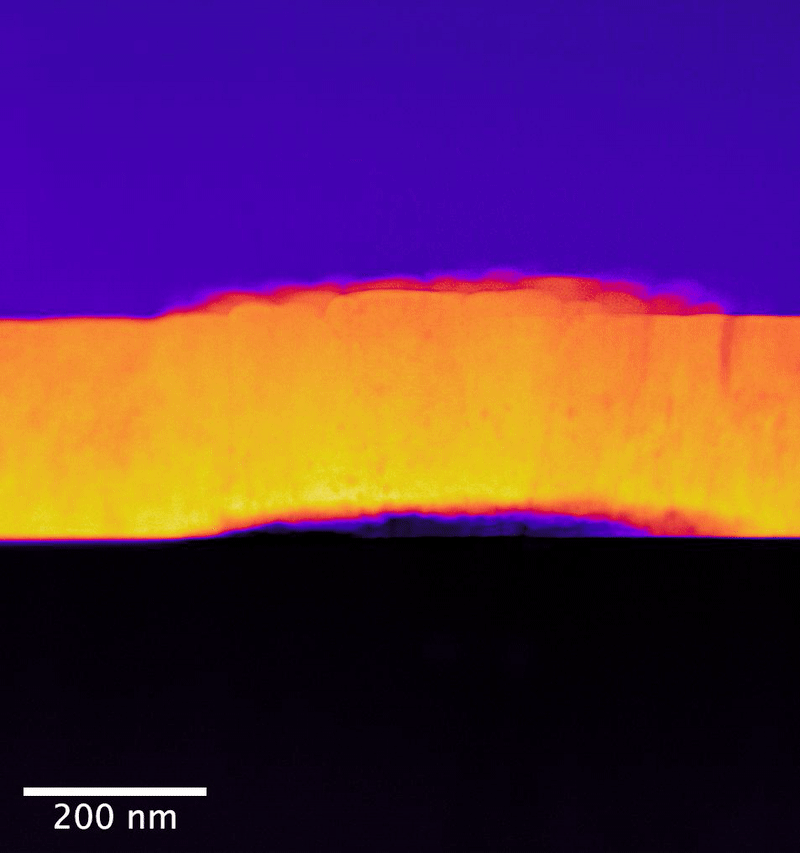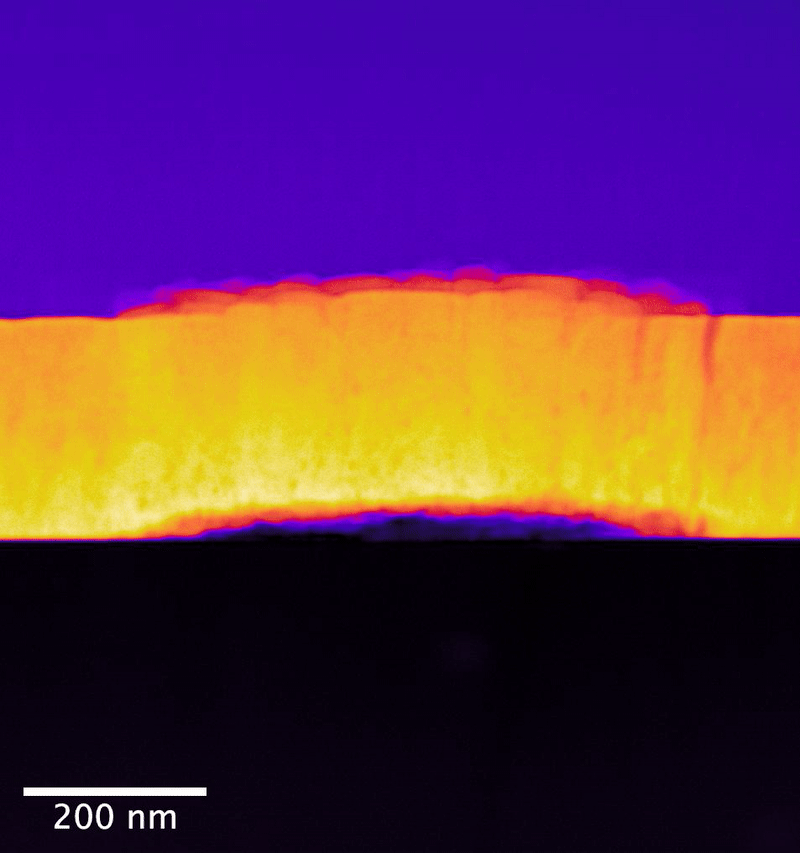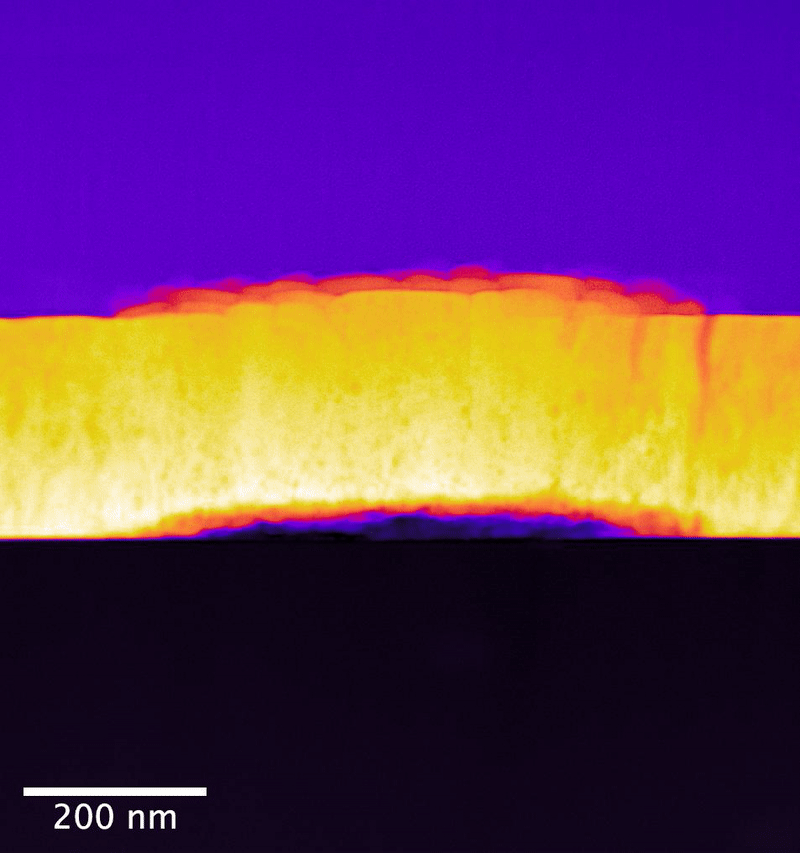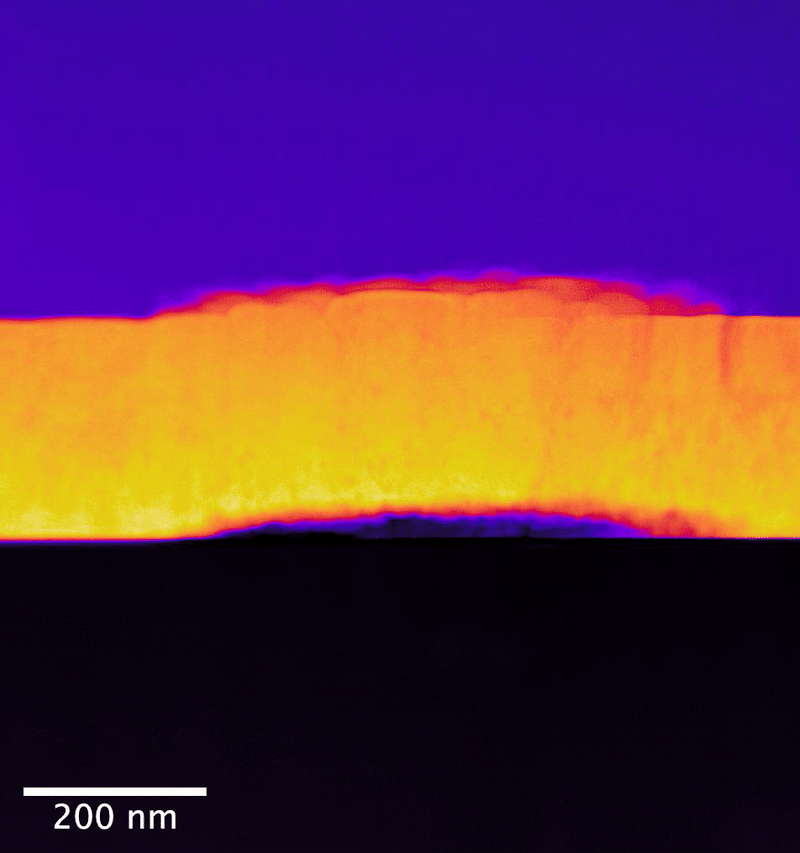
In the study, researchers from the University of Cambridge collaborated with PNNL scientists to explore UO2 samples exposed to controlled anoxic corrosion using PNNL's flagship instrumentation in the Radiochemical Processing Laboratory's Radiological Microscopy Suite. Also called the "quiet suite," this belowground room is home to the JEOL GrandARM 300F scanning transmission electron microscope (STEM). Using aberration-corrected scanning transmission electron microscopy and electron energy loss spectroscopy (EELS), the team examined the progression of atomistic structure and defects.
The PNNL team has previously shown that EELS can map nonequilibrium pathways for oxidation in UO2 that are difficult to probe using other methods.
"Our approach provides direct information at the atomic scale to improve our models for dissolution," explains PNNL materials scientist Steven Spurgeon. In turn, better models can help make more accurate, long-term predictions regarding the fate of spent nuclear fuel under anoxic disposal conditions.

Instruments inform dissolution questions
In their study, the researchers determined that dissolution initiates at material surface grain boundaries and film cracks. Importantly, they observed no amorphous surface layer formation—or, no loss of its crystalline structure— during the dissolution process. This points to a different process for oxygen substitution. Rather, oxygen substitution occurs at sites in the surface layers of the UO2 lattice. This substitution mechanism appears to create an oxidized passivating layer, which would be responsible for the observed reduction in uranium release as a function of leaching time.
"The collaboration with PNNL provided us with unique tools to uncover a behavior that would be inaccessible by other means," says co-author Prof. Ian Farnan of Cambridge. "Through our shared expertise, we were able to show how subtle changes in the surface chemistry of used nuclear fuel can control its dissolution and the release of radioactive elements to the environment—a fundamental requirement for safe disposal."
The findings from the study are reported in the team's paper, "An Atomic-Scale Understanding of UO2 Surface Evolution During Anoxic Dissolution," published in ACS Applied Materials & Interfaces.
More information: Aleksej J. Popel et al. An Atomic-Scale Understanding of UO2 Surface Evolution during Anoxic Dissolution, ACS Applied Materials & Interfaces (2020). DOI: 10.1021/acsami.0c09611
Journal information: ACS Applied Materials and Interfaces
Provided by Pacific Northwest National Laboratory