Sandwich catalysts offer higher activity and durability
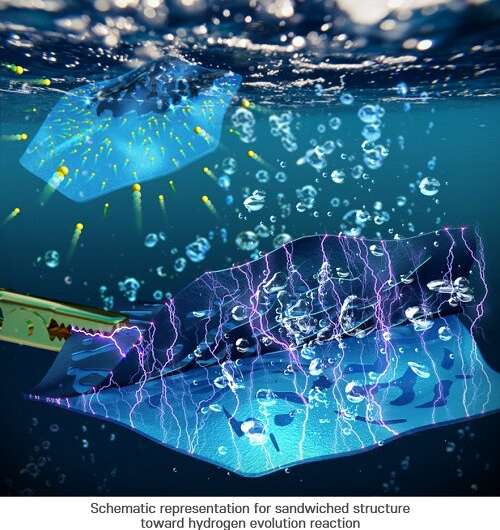
The sandwich is a food concocted by the 18th-centutry nobles to play card games uninterrupted. Meat or vegetables were layered then tucked between bread to be eaten quickly while engaged in the game. This efficient food also delivered ample calories and nutrition. A POSTECH research team has discovered that layering like the sandwich is an excellent way to obtain hydrogen energy, an alternative energy source for fossil fuels.
The research team led by Professor In Su Lee, SunWoo Jang, a student in the MS/Ph.D. integrated program, and Dr. Soumen Dutta of the Department of Chemistry at POSTECH have together developed a sandwich structured catalyst that can efficiently generate hydrogen energy by activating water electrolysis. The research findings were recently published in the American Chemical Society's international journal ACS Nano.
Hydrogen fuel cells are eco-friendly power-generating devices that generate electricity using chemical reactions that produce water (H2O) from oxygen (O2) and hydrogen (H2). With the recent release of hydrogen-powered vehicles and the spread of hydrogen fuel cells in households, hydrogen is widely seen as the next-generation energy source to replace fossil fuels. The way water is decomposed and hydrogen is produced using surplus current obtained from solar or wind power is considered the easiest and most eco-friendly way to produce high purity hydrogen fuel in large quantities. However, this method has a disadvantage of being low in production efficiency and high in cost. In order to lower the unit price of hydrogen fuel produced through water electrolysis, it is necessary to develop highly active and stable electrochemical catalysts that can maximize hydrogen generation efficiency.
-
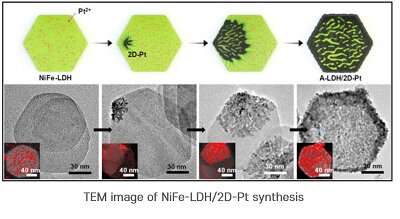
TEM image of NiFe-LDH/2D-Pt synthesis. Credit: In Su Lee (POSTECH) -
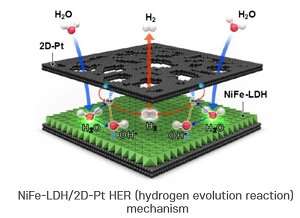
NiFe-LDH/2D-Pt HER (hydrogen evolution reaction) mechanism. Credit: In Su Lee (POSTECH)
Platinum (Pt) has been considered the most suitable catalyst for hydrogen-producing reactions, but its low affinity for water molecules and the resulting slow rate of water electrolysis make it difficult to apply to commercial processes that take place under alkaline electrolyte conditions. To make up for these limitations, many attempts have been made to combine metal-sulfide with platinum nanoparticles that promote water electrolysis, but the unstable nature of platinum/metallic-sulfide surfaces poses another weakness that significantly reduces the durability of catalysts.
In response, the research team designed a two-dimensional form of platinum/metal-hydroxide interface to improve the efficiency and durability of catalysts at the same time. In an original technique to grow a platinum layer of about 1 nm on the surface of nickel/iron-double-hydroxide(LDH), which is several nanometers thick, 2-D-2-D nanohybrid materials in the form of sandwiches containing 2-D-nickel/iron-hydroxide-nano plates have been successfully synthesized.
The synthesized sandwich catalyst has a synergistic catalytic effect between metal-hydroxide and platinum, which are in close contact across a wide 2-D-2-D interface. At this time, it shows more than 6 times the activity of the conventional catalytic material (20%-Pt/C), and maintains stable catalytic function without decreasing activity even in the hydrogen-producing water electrolysis for more than 50 hours.
"Sandwich catalysts have the highest alkali solution hydrogen-producing catalytic activity among substances that do not use carbon supports, but are significantly more durable than similar electrochemical catalysts that are stable for just three to five hours," said Professor In Su Lee who led the research. "We expect them to be applied to developing a cost-effective process for hydrogen production."
More information: Sun Woo Jang et al, Holey Pt Nanosheets on NiFe-Hydroxide Laminates: Synergistically Enhanced Electrocatalytic 2D Interface toward Hydrogen Evolution Reaction, ACS Nano (2020). DOI: 10.1021/acsnano.0c04628
Journal information: ACS Nano
Provided by Pohang University of Science & Technology (POSTECH)