Arabidopsis thaliana. Credit: Wikipedia.
A team of scientists at the Nagoya University Institute of Transformative Bio-Molecules (WPI-ITbM) have developed a method for visualizing microtubule dynamics and cell membrane protein endocytosis in living plant cells, an important step forward in plant cell biology.
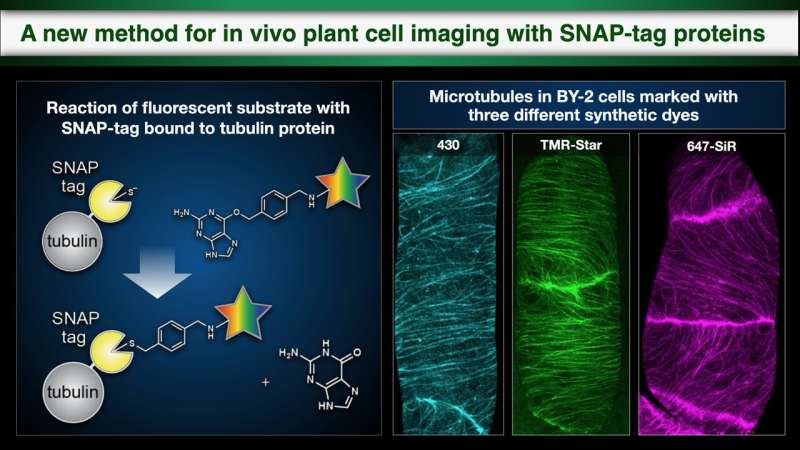
SNAP-tag visualization of in vivo protein dynamics, a method which binds dyes to proteins to allow fluorescent imaging, has made a wide range of contributions to medical and biological study, for example in cancer research. However, live cell imaging using SNAP-tag with synthetic dyes in plant science has been impossible, as synthetic dyes are unable to reach the target proteins due to the presence of the cell wall in plant cells.
In this study, the research team demonstrated that it was possible to perform live cell imaging using SNAP-tag even in plants, using three types of synthetic dyes which bond to the SNAP-tag to mark microtubules, part of the cytoskeleton. They were able to use a particular dye that does not permeate the cell membrane and fluoresces only when it bonds with SNAP-tag to exclusively mark the auxin transporters in the cell membrane, visualizing the process of membrane proteins being taken into the cell (endocytosis) after they had been marked. Thus, they were able to clearly differentiate between transporter proteins which had been newly synthesized inside the cell and those taken into the cell by endocytosis.They then used tobacco cells to find out which of 31 different dyes were able to enter the cell. Interestingly, it was found that 23 out of these 31 were taken into the tobacco cells, that the majority of them could be used with SNAP-tag to mark cytosolic components in plant cells, and that those which could not permeate the cell membrane could be used to mark membrane proteins outside the cell.
This study provides a new technique for the fluorescent marking of proteins in plant cells, and represent an important step forward in plant cell biology research. It is expected to find use in superresolution imaging using extremely light stable markers, and techniques for determining place- and time-specific pH and Ca2+ levels.
A new technique for the fluorescent marking of proteins in plant cells which represents an important step forward in plant cell biology research. Credit: Issey Takahashi
More information: Ryu Iwatate et al, Covalent Self-labeling of Tagged Proteins with Chemical Fluorescent Dyes in BY-2 Cells and Arabidopsis Seedlings, The Plant Cell (2020). DOI: 10.1105/tpc.20.00439
Journal information: Plant Cell