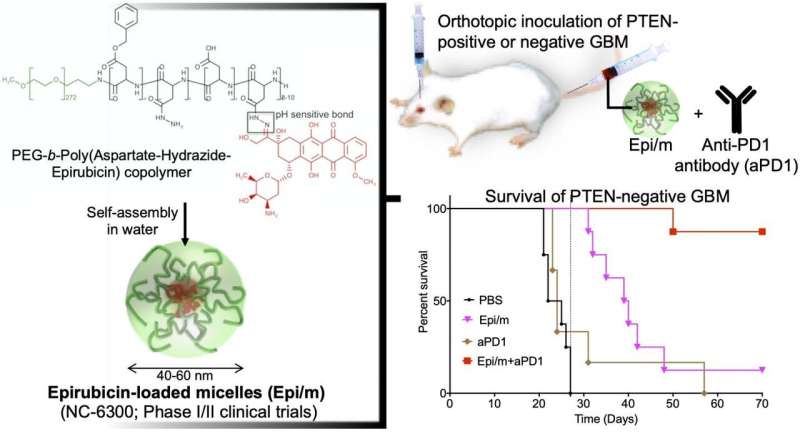
Left: Hydrophobic epirubisin is conjugated to one end of hydrophilic polyethylene glycol (PEG) chain with aspartate-hydrazide as a linker. In water, this molecule is self-assembled to form nano-micelles (Epi/m). Upper right: PTEN(+) or PTEN(-) GBM was transplanted into the brain of mice, and Epi/m and anti-PD1 antibody (aPD1) were administered through the tail vein to evaluate survival period. Bottom right: Comparison of survival period in case of PTEN(-)GBM. PBS (phosphate buffer solution) was administered to the control group. As a result, none of the control group (black) could survive more than 30 days (8/8). Epi/m alone group (pink) died gradually after 30 days, half (4/8) in 40 days, and 7/8 by 50 days. aPD1 alone (brown) killed 6/7 within 30 days. In contrast, using Epi/m+aPD1 (red), 1/8 died 50 days later, but 7/8 were alive after 3 months even. Credit: 2020 Innovation Center of NanoMedicine
A nanomedicine-based strategy for chemo-immunotherapy (CIT) of glioblastoma (GBM), which has the worst prognosis among brain tumors, was successfully developed. In vivo experiments demonstrated that the combined use of epirubicin-encapsulating nano-micelles (Epi/m) with immune checkpoint inhibitors (ICI) eradicated PTEN-negative GBM, which is highly resistant to ICI alone. Due to the synergistic effects of Epi/m plus ICI combination, the number of tumor-infiltrating T cells (TIL) and other antitumor immune cells significantly increased to kill cancer cells effectively.
On the other hand, intratumoral bone marrow-derived immunosuppressive cells (MDSC), which interfere with the immune response, were significantly reduced. The CIT also provided robust immunological memory effects against the tumors, which effectively rejected newly implanted PTEN-negative GBM cells in the brain. While free epirubicin can cause damage to organs, including hematopoietic organs especially, our nanomedicine strategy significantly reduced these side effects, improving the immune response. Epi/m has already advanced into clinical trials for other cancer types, and this CIT strategy could be expected to be translated to clinical evaluation in the future. These results have been published in ACS Nano on August 6 by the American Chemical Society.
The Innovation Center of Nanomedicine (Director: Prof. Kazunori Kataoka, Location: Kawasaki-City, Abbreviation: iCONM) announced that a new therapeutic option for glioblastoma (GBM) was demonstrated in mice, in a collaboration study with the Department of Bioengineering, Graduate School of Engineering, The University of Tokyo. GBM is a brain tumor with extremely rapid progression and poor prognosis (5-year survival rate: 10.1%). Although several compounds are being evaluated in clinical studies, there is no therapeutic option to significantly improve the survival period. In particular, patients with abnormalities in the PTEN gene, one of the cancer suppressor genes, are highly resistant to currently available therapies and have high medical needs.
In general, immune checkpoint inhibitors (ICIs) are considered to ineffective against GBM, as GBM is immunosuppressive with low T cell infiltration. In the method presented in this paper, iCONM's nano-drug delivery technology allows selective tumor accumulation of epirubicin, which causes immunogenic cell death (ICD), to tumor tissues, thereby, causing ICD locally for synergizing with ICI. As a result, this nanomedicine-based chemo-immunotherapy (CIT) was effective in mice transplanted with GBM in the brain (hereinafter referred to as mouse GBM model), and succeeded in significantly prolonging mice survival. The combination of the epirubicin-loaded nano-micelles treated mice showed high infiltration of cytotoxic T cells (TIL) and decreased bone marrow-derived immunosuppressive cells (MDSC). Eventually suppression of the immune checkpoint function was observed.
Mutations in the PTEN gene occur frequently in GBM, resulting in immunosuppressive pathways that promote the resistance to ICIs. Thus, while ICIs eradicated 40% of tumors in a mouse GBM model in which the PTEN gene is normal, in a model in which the PTEN gene was knocked-out, ICIs were unable to extend mice survival. At the cellular level, it was found that PTEN-deficient cells (CT2A-luc) expressed approximately 5-fold more PDL1 than that of normal cells, which is probably connected to the therapeutic resistance with ICI. As epirubicin have shown the ability to suppress PDL1 expression in tumors, such as breast cancer, it would be possible to decrease PDL1 levels of GBM if sufficient amount of epirubicin can be delivered into GBM lesions. Thus, CIT using nanomicelles containing epirubicin (Epi/m) in combination of ICI were used for enhancing the antitumor efficacy against GBM.
In a GBM model with normal PTEN expression (GL261-luc), Epi/m (5 mg/kg on Epi basis) plus anti-PD1 antibodies (5 mg/kg) resulted in the survival of all mice for more than 70 days, with a remarkable extension of survival time. In this model, PBS-treated mice died within 30 days, mice treated with anti-PD1 antibodies alone (5 mg/kg) allowed 40% of mice to survive for at least 70 days, and Epi/m (5 mg/kg of Epi basis) resulted 80% of mice survival for more than 70 days. In contrast, in the PTEN-deficient model (CT2A-luc), Epi/m (5 mg/kg on Epi basis) plus anti-PD1 antibodies (5 mg/kg) resulted in only 30% of mice survival for more than 70 days, and no clear survival effect could be confirmed for the other control groups. When the dose was increased to 15 mg/kg of Epi/m (in Epi basis) and combined with anti-PD1 antibodies (5 mg/kg), 90% of mice were able to survive for more than 70 days, remarkably prolonging mice survival.
More information: Hiroaki Kinoh et al, Translational Nanomedicine Boosts Anti-PD1 Therapy to Eradicate Orthotopic PTEN-Negative Glioblastoma, ACS Nano (2020). DOI: 10.1021/acsnano.0c03386
Journal information: ACS Nano
Provided by Innovation Center of NanoMedicine