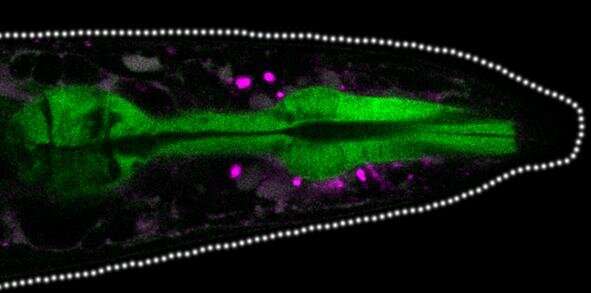
Protein aggregates (magenta dots) accumulate between tissues in the head of the worm. Credit: University of Tübingen
With increasing age, and especially in neurodegenerative diseases such as Alzheimer's, proteins tend to misfold and aggregate into harmful deposits both inside and outside cells. Secreted proteins play an important role in regulating body functions and fighting infections. Now a collaborative team at the University of Tübingen has discovered mechanisms to stop secreted proteins from forming deposits outside the cell. The team is headed by Della David, who researches aging, at the Interfaculty Institute of Biochemistry and at the German Center for Neurodegenerative Diseases (DZNE). The findings indicate that keeping proteins in shape in body fluids helps to combat both aging and infections. The study has been published in the latest edition of Nature.
The body uses proteins as building blocks in cells—but in the form of enzymes, for example, proteins are also responsible for many metabolic processes. For this, the long amino acid chains which go to make up proteins must be folded into the correct three-dimensional shape. "Decades of research have focused on protein quality control mechanisms inside the cell which work to avoid harmful protein aggregation," says Della David. But such misfolded deposits also occur outside of cells. Little was known about how they are regulated because the process is very difficult to investigate in experimental animals such as mice.
A new model for extracellular protein aggregation
In order to identify extracellular regulators, the team created a new model for extracellular protein aggregation using the tiny worm Caenorhabditis elegans. David and her colleagues found 57 extracellular regulators of protein aggregation in C. elegans. Working with Martin Haslbeck at the Technical University of Munich, they identified the first extracellular regulator that binds to and stabilizes misfolded secreted proteins in worms. "We knew that better protein quality control inside cells helps the animals to live longer. Now we have shown that better protein quality control outside the cell does too," says David. "Intriguingly, the worms mobilize extracellular regulators in response to infections by pathogens," she says. The study's first author Ivan Gallotta adds: "We were really surprised to find that animals with better extracellular protein quality control could survive over 30 percent longer during a pathogenic attack." In collaboration with Ralf Sommer at the Max Planck Institute for Developmental Biology in Tübingen, the researchers found that extracellular regulators boosted the animals' immune response.
"Many mechanisms and protein functions are shared between worms and humans," says Maximilian Peters from the Hebrew University of Jerusalem, who also participated in the study. He identified the human proteins with the highest similarities to the worm extracellular regulators. "Our next goal is to determine if these regulators could be active against extracellular amyloid-beta protein aggregation, which is found in the brains of patients with Alzheimer's disease," says David. With her findings, she will seek to open up new avenues in the quest for effective treatments for Alzheimer's disease. "Learning more about extracellular protein quality control may also lead to a better understanding of how to promote healthy aging and protect us against infections," she says.
More information: Ivan Gallotta et al. Extracellular proteostasis prevents aggregation during pathogenic attack, Nature (2020). DOI: 10.1038/s41586-020-2461-z
Journal information: Nature
Provided by University of Tübingen