S-glutathionylation of human-inducible Hsp70 reveals regulatory mechanism involving C-terminal α-helical lid
Heat shock protein 70 (Hsp70) proteins are a family of ancient and conserved molecular chaperones. They play an essential role in maintaining protein homeostasis, including facilitating protein folding and degradation, preventing protein aggregation, and participating in the stress response. Disruption of the cellular quality control machinery is associated with aging, cancer and neurodegenerative diseases.
The role of post-translational modifications (PTMs) in regulating the functions of Hsp70 is an emerging field of research. Although oxidative stress can be harmful, redox variation is a natural feature of the cellular environment and facilitates signal transduction for important physiological activities. Cysteine modifications of proteins provide the main means for redox signal transfer. Glutathionylation is a reversible modification of cysteine residues in proteins, which can protect proteins from irreversible oxidation, and can also play a role in signal transduction.
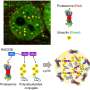
In this study, glutathionylation of different Hsp70 family members was detected in HeLa cells and the modification sites were ascertained by mass spectrometry by researchers from Prof. Sarah Perrett and Prof. Chen Chang's group at the Institute of Biophysics of the Chinese Academy of Sciences.
Focusing on stress inducible Hsp70 HspA1A (hHsp70), the detailed structural mechanism of how glutathionylation affects protein activity and protein-protein interactions was investigated.
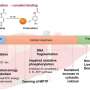
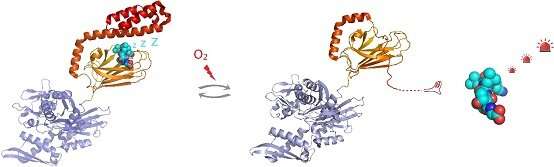
In vivo detection showed that each of the five cysteine residues of hHsp70 can undergo glutathionylation. In vitro experiments revealed that modification of cysteines in the nucleotide-binding domain of hHsp70 is prevented by nucleotide binding, but that Cys-574 and Cys-603, located in the C-terminal α-helical lid of the substrate-binding domain, can undergo glutathionylation in both the presence and absence of nucleotide.
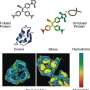
By solving the NMR structure of the glutathionylated form of the hHsp70 SBD, the structural basis for the functional changes was demonstrated. Glutathionylation of these cysteine residues results in unfolding of the α-helical lid structure. The unfolded region mimics substrate by binding to and blocking the substrate-binding site, thereby promoting intrinsic ATPase activity, and competing with binding of external substrates. This leads to a reduction in its ability to bind other substrate proteins, such as the heat shock factor Hsf1.
These results indicate not only that cysteine modification can alter the structure and function of hHsp70, but also that hHsp70 can transfer redox information to its clients.
This study was published in the Journal of Biological Chemistry on 12 June 2020.
More information: Jie Yang et al. S-Glutathionylation of human inducible Hsp70 reveals a regulatory mechanism involving the C-terminal α-helical lid, Journal of Biological Chemistry (2020). DOI: 10.1074/jbc.RA119.012372
Journal information: Journal of Biological Chemistry
Provided by Chinese Academy of Sciences