Nanocomposites with rich oxygen vacancies promote sensitive electroanalysis of Hg(II)
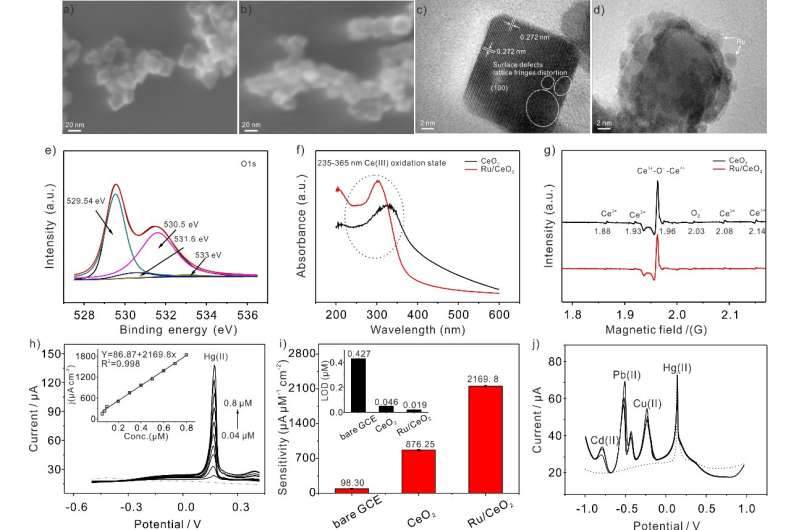
Recently, Yang Meng and his colleagues from the Institute of Solid State Physics, Hefei Institutes of Physical Science reported a sensitive electrochemical sensing performance of Ru-loaded single-crystalline (100) CeO2 nanocomposites toward heavy metal ions (e.g., Hg(II)).
Metal oxides nanomaterials are limited in electrochemical detection of heavy metals due to their poor conductivity and less active sites, which hinders electron transport and reduces the redox rate of heavy metal ions (HMIs) on the surface, making it difficult to achieve sensitive and accurate detection of trace heavy metal pollution.
Therefore, improving the sensitivity of metal oxides nanomaterials to detect HMIs by increasing the conductivity and enriching the surface active sites has become the focus of scientists' research.
To tackle this problem, the research team developed Ru-loaded cerium dioxide nanocubes (Ru/CeO2) with rich oxygen vacancies (OVs) to construct electrochemical sensing interface, which was used to detect Hg(II).
In addition to the novel fabrication, they also explored the possible mechanism of electrochemical signal enhancement through a series of electrochemical experiments, X-ray photoelectron spectroscopy (XPS) and electron paramagnetic resonance (EPR), etc.
The research results indicated that the predominantly exposed highly active (100) crystal facets and abundant OVs on the surface of CeO2 nanocubes, as well as Ru nanoparticles with excellent metal activity on the surface of CeO2 nanocubes, which may provide a large amount of reactive oxygen species and active sites, and enhance the conductivity of the Ru/CeO2 nanocomposites, then to achieve outstanding electrochemical properties by promoting the redox reaction of Hg(II).
Besides, the high anti-interference detection of Hg(II) in the presence of other HIMs was realized during their lab work.
Moreover, the accurate analysis of real water samples by Ru/CeO2 nanocomposites demonstrates the great potential of their proposed method for electrochemical detection of Hg(II).
These findings not only expand the electrochemical sensing applications of pure semiconductors, but also shed new light on the new way of investigating atom-level electrochemical behaviors of semiconductors by surface electronic state modulation.
More information: Yu-Feng Sun et al. Ruthenium-loaded cerium dioxide nanocomposites with rich oxygen vacancies promoted the highly sensitive electrochemical detection of Hg(II), Sensors and Actuators B: Chemical (2020). DOI: 10.1016/j.snb.2020.128355
Provided by Chinese Academy of Sciences




















