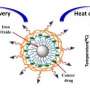
Thermosensitive liposome-coated gold nanocages with DOX loading (LAD) for photothermally triggered drug release and breaking chemoresistance. Credit: SIAT
Chemotherapeutic drugs are the cornerstone of the treatment for numerous malignancies. However, current chemotherapy is still far from being satisfactory due to severe side effects of the chemotherapeutic agents and drug resistance of cancer cells. Thus, constructing an ideal chemotherapeutic strategy to reverse drug resistance is needed.
Researchers from the Shenzhen Institutes of Advanced Technology (SIAT) of the Chinese Academy of Sciences have developed a smart nanosystem with enhanced curative efficacy and lower side effects. The study was published in Journal of Controlled Release.
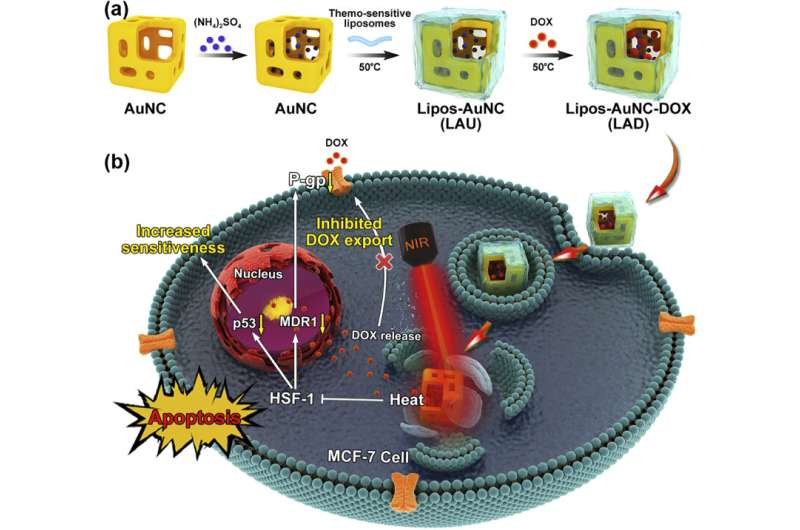
This smart nanosystem consists of thermosensitive liposome-coated gold nanocages with doxorubicin (DOX) loading (LAD) for near-infrared (NIR)-triggered drug release and chemo-photothermal combination therapy for breast cancer.
The biocompatible liposome coating facilitated the cellular uptake of LAD and avoided drug leakage during circulation. More importantly, LAD exhibited controllable photothermal conversion and produced mild heat under NIR irradiation, which not only triggered DOX release and transferred DOX from lysosome to nucleus, but also elicited the mild heat cell killing effect to improve the curative efficiency.
Further study revealed that mild heat could reverse drug resistance by down-regulation of the chemoresistance-related markers, and inhibited DOX export and increased drug sensitivity, thereby prominently increasing the anticancer efficiency.
This smart, versatile LAD nanoplatform is promising for the fields of controlled drug release and chemo-photothermal combination therapy.
More information: Huamei He et al. Smart gold nanocages for mild heat-triggered drug release and breaking chemoresistance, Journal of Controlled Release (2020). DOI: 10.1016/j.jconrel.2020.04.029
Journal information: Journal of Controlled Release
Provided by Chinese Academy of Sciences