Scientists propose strategy for site-selective chiral drug synthesis in living systems
Enantioselectivity plays an important role in the pharmacological and toxicological processes of chiral drugs.
In chiral drug synthesis that uses bio-orthogonal chemistry within complex biological milieu, asymmetric transfer hydrogenation (ATH), which utilizes safe hydrogen donors instead of high pressure H2, has been gaining popularity and has been performed in living cells.
Prof. Qu Xiaogang from the Changchun Institute of Applied Chemistry (CIAC) of the Chinese Academy of Sciences and his colleagues recently presented a novel strategy using a neutrophil-directed ATH reaction to achieve site-selective chiral drug synthesis in living systems.
The study was published in Chem on June 25.
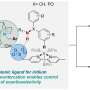
The researchers first constructed inflammation-targeted chiral catalysts by preparing Pd-immobilized mesoporous silica nanoparticles (MSN-Pd).
Cinchona alkaloid enantiomers were individually adsorbed on the MSN-Pd catalyst to introduce enantioselectivity.
A neutrophil membrane was subsequently coated onto the MSN-Pd chiral catalysts, creating the MSN-Pd/CD@Neu catalyst, which possessed inflammation-targeting ability inherited from the neutrophils.
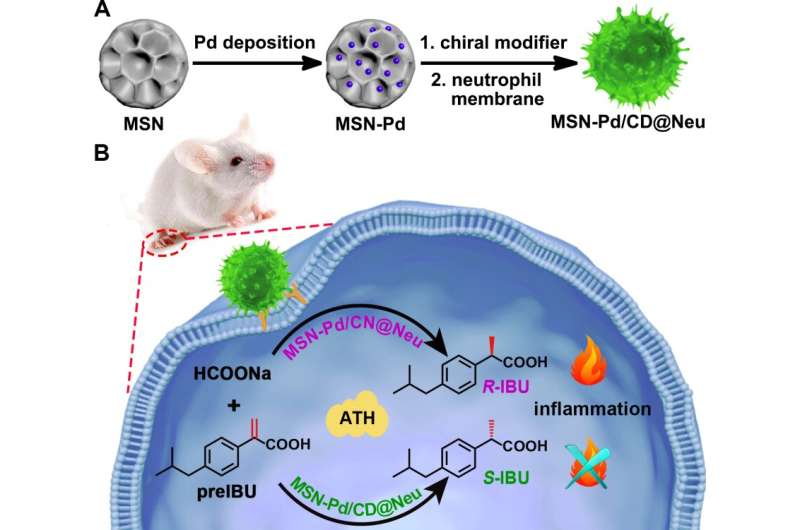
The next stage of research involved using MSN-Pd/CD@Neu to catalyze an intracellular conversion involving the precursors of Ibuprofen (IBU).
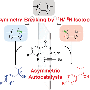
IBU is a drug well-known for relieving inflammation, fever and pain. The pharmacological activity of IBU is mainly attributed to the S-enantiomer while the R-enantiomer is thought to be potentially harmful. For this reason, it is important to be able to control the catalysis of IBU into its enantiomers.
In this study, the conversion of the precursor molecule of IBU (pre-IBU) to S-IBU was realized by using a targeted ATH bio-orthogonal reaction in a mouse paw model, with HCOONa as the hydrogen donor.
Compared with the controls, the MSN-Pd/CD@Neu catalyst simultaneously exhibited inflammation-targeted capability and enantioselectivity as part of its anti-inflammatory action.
In situ synthesized IBU can ameliorate lipopolysaccharide (LPS)-triggered acute inflammation, pyrexia and tissue edema.
This research opens a new avenue for chiral drug synthesis activated on the designed target in living systems. It also holds promise for wide application of targeted bioorthogonal chemistry, especially in enantioselective drug synthesis.
More information: Zhi Du et al, Neutrophil-Membrane-Directed Bioorthogonal Synthesis of Inflammation-Targeting Chiral Drugs, Chem (2020). DOI: 10.1016/j.chempr.2020.06.002
Journal information: Chem
Provided by Chinese Academy of Sciences