Background and microfluidic redox-neutral electrochemistry (μRN-eChem). Credit: Science (2020). DOI: 10.1126/science.aba3823
A team of chemists and engineers at MIT has found a new way to apply microfluidic electrochemical technologies to single-electron transfer (SET) redox-neutral reactions. In their paper published in the journal Science, the group describes introducing a microfluidic redox-neutral electrochemistry to the platform and explain why they believe it has broad applicability to SET chemistry. Jian-Quan Liu, Andrey Shatskiy and Markus Kärkäs have published a Perspective piece in the same journal issue outlining the recent history of photoredox catalysis and electrosynthesis, and explaining why it is an important component of the search for new synthetic methods—they also outline the work by the team at MIT.
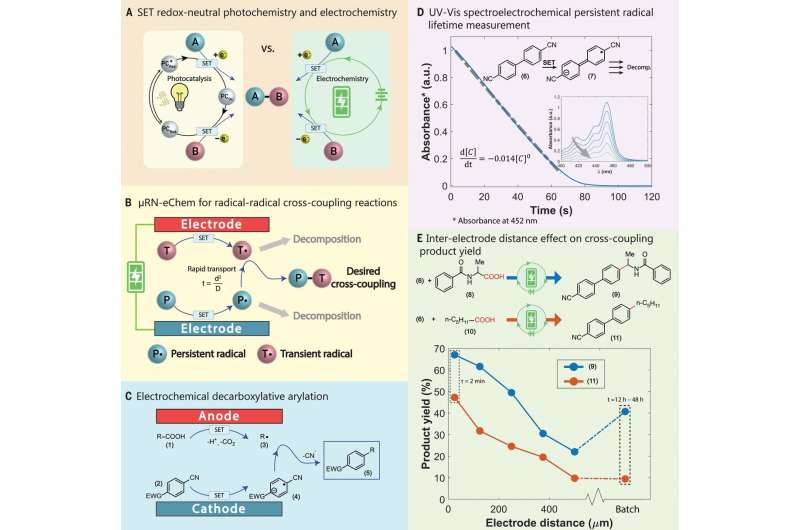
Over the past several years, chemists have been looking into new ways to use visible-light photocatalysis as part of organic synthesis efforts. And while such efforts have proved fruitful in a variety of ways, they have also run into serious limitations—the need for retuning of redox potentials, for example, and the high expense involved when using transition-metal photocatalysts. There have also been incompatibility issues and the need to remove transition metals. Such issues have led chemists to turn to electrosynthesis, which, as its name implies, is a type of synthesis that is aided by electricity. The researchers note that in many ways, electrosynthesis is an excellent choice for use in radical coupling; in principle, it is both simpler and cheaper—a given precursor is oxidized near the anode, while its counterpart is reduced near the cathode. The big problem has been one or the other partners losing stability before they meet somewhere in the center.
In this new effort, the team at MIT has found a way around this problem by placing the components near one another in a microfluid platform. In their setup, the oxidation and reduction reactions take place only on the surfaces of the electrodes where the materials used (dicyanobenzene with a variety of partners) can meet rapidly and react. Liu, Shatskiy and Kärkäs suggest this new approach should provide chemists a powerful new tool for use in work with redox-neutral free-radical reactions.
More information: Yiming Mo et al. Microfluidic electrochemistry for single-electron transfer redox-neutral reactions, Science (2020). DOI: 10.1126/science.aba3823
Journal information: Science
© 2020 Science X Network