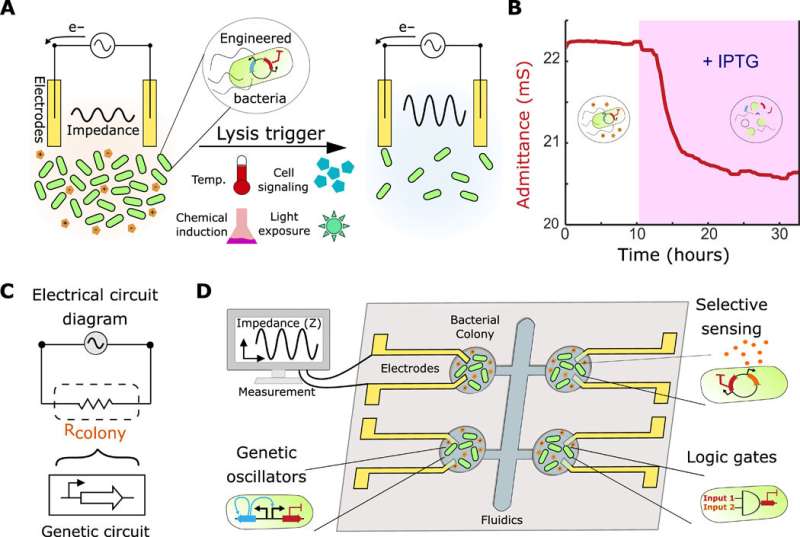
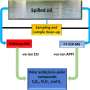
Interfacing genetic circuits with electrical measurement. (A) Schematic of the approach using a culture of bacteria with a killing gene as the circuit output in contact with inert gold electrodes. The impedance of the culture reduces during growth (left) and increases upon the induction of bacterial death due to the clearance of charged metabolites (right). (B) Profile of the admittance (red), which is the inverse of impedance, using an IPTG-inducible lysis construct (pE35GFP) in an electro-chemostat device. The pink shaded region represents induction of lysis with 1 mM IPTG in the medium. (C) Schematic of the equivalent electrical circuit for our strategy using an alternating input voltage. The bacterial population is simplified to a resistor, which is controlled by a genetic circuit. (D) Schematic showing a microelectronic platform to interface between engineered bacteria and electronics. Several chambers may contain unique genetic circuits, connected via electrodes to an impedance output system. Credit: Science Advances, doi: 10.1126/sciadv.aaz8344
The ability to detect the growth of a bacterial colony by monitoring changes in impedance (a measure of resistance) across time reflects the impressive scientific progress connecting bacterial behavior with electrodes via synthetic biology. In a new report, M. Omar Din and a research team at the BioCircuits Institute, department of bioengineering and molecular biology at the University of California, San Diego, U.S., interfaced synthetic biology with microelectronics using engineered population dynamics. They regulated the accumulation of charged metabolites and electrically detected the bacterial response to heavy metals using a population control circuit. During the experiments, the scientists used a synchronized genetic oscillator and obtained an oscillatory impedance profile with engineered bacteria. They miniaturized the array of electrodes to form bacterial integrated circuits and demonstrated their applicability as an interface with genetic circuits. The new approach is now published in Science Advances and will pave the way for advances in synthetic biology, analytical chemistry, and microelectronics.
Synthetic biologists can advance our understanding of biology by investigating gene expression in biological organisms using diverse monitoring methods to investigate gene circuits in the lab. Such methods are critically important in synthetic biology to characterize gene circuit behavior and accomplish tasks such as sensing. Most techniques rely on fluorescent reporter genes that convert biological responses into a detectable signal, but they generally rely on sophisticated optics, which pose relatively intrinsic challenges due to the presence of background signals and toxicity. Advances in microelectronics provide a promising alternative to understand smart analytical tools, where biological molecules can be useful for sensing and mechanical actuation. Despite their higher selectivity such methods are limited by detection techniques that require expensive purification procedures, complex immobilization and labeling steps.
Time-lapse microscopy showing transmitted light of the synchronized lysis circuit (SLC) at 4X magnification in a bIC device. Bacterial growth chambers on the top and bottom are connected to gold electrodes and images were taken every 10 min. Credit: Science Advances, doi: 10.1126/sciadv.aaz8344
Din et al. engineered bacterial circuits to control the release of ionic species through cell growth and death and transduced (converted) the gene circuit output to an electronic signal. They inserted gold electrodes to measure the AC impedance after applying a small sinusoidal voltage to the bacterial populations—where the measurements naturally included resistive and capacitive effects. When the optimized bacteria grew to a stable density in the continuous culture, Din et al. triggered the lysis (degradation) gene of the population through chemical induction, and induced bacterial lysis to enable marked changes in the conductive properties of the culture. The engineered bacteria were able to regulate the flow of electrical current in the circuit for their role as bacterial conductors (or resistors) while the team controlled the electrical properties via genetic modulations, which in turn controlled the bacterial population dynamics.
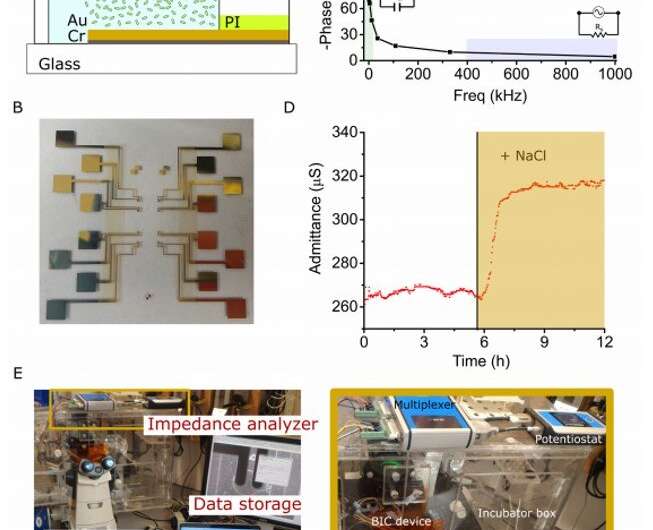
The bIC set-up and performance. (A) Schematic of fabrication of electrode's in bIC on glass consisting in a sequential deposition of conductive chrome and gold layers and a final spin of a polyimide insulator layer. (B) Picture of the lithography fabricated electrodes on glass. (C) Bode plot in bIC for LB media and correspondent equivalent circuits at different frequencies. (D) Admittance response of microfluidic electrodes using LB, induced with 100 μM NaCl (shadowed yellow) using same procedure than with multiple bICs. (E) Picture of the complete set-up used for bIC data acquisition including microscope and impedance analyzer. Photo credit: M. O. Din, UCSD. Credit: Science Advances, doi: 10.1126/sciadv.aaz8344
Researchers had previously investigated the dynamics of gene-circuits with well-defined submillimeter-scale bacterial colonies. To study the dynamic behavior of robust populations in a macroscale continuous culturing system, Din et al. developed a customized millimeter-scale chemostat with disposable gold electrodes in contact with the cell culture. The setup allowed in-house real-time data collection to directly correlate the culture density and impedance in an electro-chemostat system. In this experiment, the team induced cell lysis using a heavy metal toxin and selected an arsenic-sensitive strain. They noted a fast lysis trigger with arsenic, but the bacterial strains did not deconstruct in response to related toxins such as copper. As further control, they used a copper-sensitive strain to induce lysis in the presence of copper and experimentally discriminated between the two related heavy metal toxins.
Aside from analytical capabilities, the team also implemented oscillatory synthetic circuits as synchronized lysis circuits (SLC). The bacterial genetic circuit contained a common promotor (known as pLuxI) that drove the expression of the gene LuxI, which produced the quorum sensing molecule acyl homoserine lactone (AHL) that further activated the promoter by binding to the activator LuxR. The AHL therefore provided an intercellular synchronization mechanism to synchronize lysis at the population level and the setup allowed cyclic repetition of AHL production, oscillatory growth, and lysis behavior. Din et al. measured the synchronized lysis circuit strain using the electro-chemostat and noted the corresponding behavior in the signal in time.
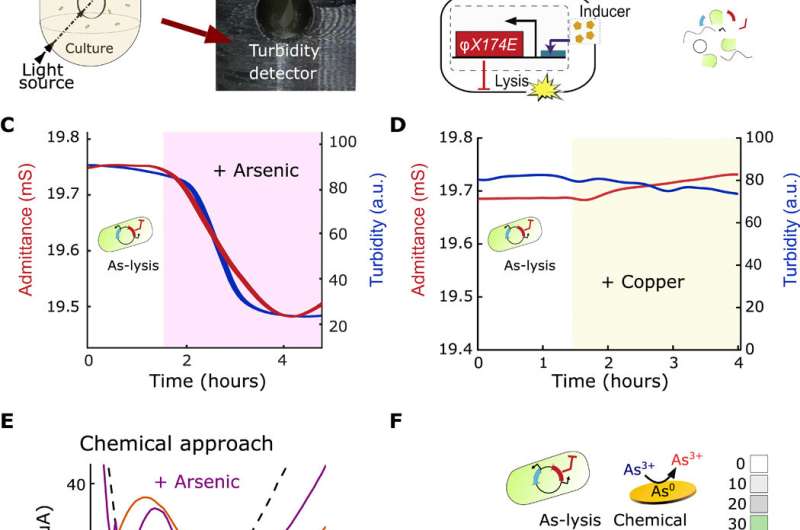
Interfacing bacterial circuits for impedimetric sensing. (A) Schematic and picture of electrochemostat components included in a 3D-printed holder containing a culture tube, in which the disposable electrode consisting of gold counter/reference (C/RE) and working (WE) electrodes are immersed, and an external turbidimeter detector. (B) Equivalent electrical circuit for a bacterial population engineered with an inducible promoter driving the expression of the lysis gene, E, and schematic of engineered bacterial (As-lysis) death upon arsenic induction. (C) Profiles of the admittance (red) and turbidity (blue) using an arsenic-sensitive strain in an electrochemostat device. The shaded region represents the duration of 250-ppb arsenic induction. (D) Profiles for the As-lysis strain induced with 250-ppb copper. a.u., arbitrary units. (E) Square-wave voltammetry profiles for 250-ppb arsenic (purple) and 250 ppb copper (orange). Dashed line shows the signal of the HNO3 buffer (background). The oxidation potential at the maximum current intensity versus background signal is indicative of the presence of the metal. (F) Heat maps of selectivity to arsenic versus copper using our bacterial lysis and chemical approaches. Photo credit: M. O. Din, UCSD. Credit: Science Advances, doi: 10.1126/sciadv.aaz8344
The team met substantial challenges to minimally track gene expression without using fluorescent proteins and associated complex imaging equipment. To overcome this, they developed a label-free method on a microfluidic chip with multiple millimeter-scale growth chambers containing electrodes composed of conductive interlayers of gold and chrome. They seeded each of the electrode containing chambers with engineered bacteria and connected each genetic circuit output to electrochemical measurements. The team connected multiple bacterial ion circuits in parallel to measure multiple strains and measured resistive effects in the growth chambers across a range of input frequencies. The bacterial integrated chip (bIC) thereby detected gene circuit behavior by detecting impedance of ions in solution. The results showed the functionality of bIC devices as miniature platforms to electrochemically measure genetic circuit output. The work provides a simple, label-free method to measure real-time data expressed by engineered bacteria.
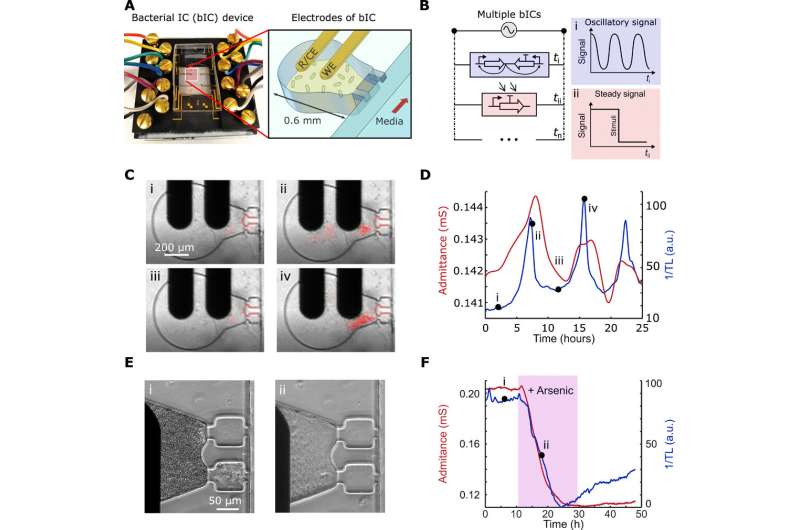
The bICs in millifluidic devices. (A) Photograph of the bIC device (left) and schematic showing the design of the growth chamber and adjoining gold electrodes. Each bIC integrates lithography-fabricated electrodes, including a reference/counter electrode (R/CE) and a WE electrode, on 0.6-mm-diameter traps where bacteria form 3D continuous cultures. (B) Schematic of the equivalent electrical circuit illustrating multiple parallel interconnecting bICs measured with a single potentiostat. The dynamics in each bIC is measured sequentially in time intervals (Δt). A device containing n unique bICs may output an oscillatory signal at ti, a steady signal at tii, and other distinct signals up to tn. (C) Images of a bIC, containing the Salmonella SLC strain, were taken using transmitted light (TL). The bacteria begin at a low cell density (i) from which they reach the quorum threshold and lyse (ii and iii), and repeat the process (iv) cell growth in red is superimposed with the original time lapses in movie S1. (D) Profiles of admittance (red line) and inverse of the TL (blue line) for the strain in (C). (E) Images of the response of the bIC with the arsenic-inducible construct in E. coli MG1655 showing the steady growth state (i) and after 250-ppb arsenic induction (ii). (F) Profiles of admittance (red line) and inverse of the TL (blue line) for the strain in (E). Photo credit: A. Martin, UCSD. Credit: Science Advances, doi: 10.1126/sciadv.aaz8344
In this way, M. Omar Din and colleagues demonstrated the ability to use engineered population control circuits with electrochemical measurements for simple and cost-effective sensing that bypassed optical requirements. They connected the bacterial sensing output directly to the electrodes without separate steps for isolation and preparation, in order to develop the biochemical sensor. The scientists used arsenic, copper and chemical agents as manual triggering factors of lysis, with potential to also include light, temperature, and bacteriophages during further experiments. Compartmentalization of bacterial colonies within the bIC will also pave the way to create and monitor large libraries for high throughput monitoring.
The approach can readily connect microelectronics with a variety of genetic circuits such as logic gates, switches, oscillators, and sensors to construct hybrid computational devices. Din et al. envision that such strategies may allow an array of bacterial colonies to process external information through genetic circuitry and transfer them through impedance for analysis on an electronic device. Such concepts can lead to bacterial integrated circuit-based environmental sensors on earth or in space and allow scientists to investigate unexplored concepts through synthetic biology.
More information: M. Omar Din et al. Interfacing gene circuits with microelectronics through engineered population dynamics, Science Advances (2020). DOI: 10.1126/sciadv.aaz8344
Owain Vaughan. Better sensing with bacteria, Nature Electronics (2018). DOI: 10.1038/s41928-018-0111-3
Yangxiaolu Cao et al. Programmable assembly of pressure sensors using pattern-forming bacteria, Nature Biotechnology (2017). DOI: 10.1038/nbt.3978
Journal information: Science Advances , Nature Electronics , Nature Biotechnology
© 2020 Science X Network