Researchers find new shape for hydrophobic molecules in water
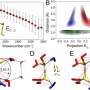
The embedding of hydrophobic molecules in water looks quite different than previously assumed. In water, hydrophobic molecules are surrounded by a two different water populations: the inner shell forms a two-dimensional network of water molecules. The next layer is formed by a second water population that is almost bulk like but forms slightly stronger hydrogen bonds to the bulk water. The assumption to date was that tetrahedral, "ice-like" water dominate in the innermost hydration shell of hydrophobic molecules. The opposite is the case. These new findings were published by the team headed by Professor Martina Havenith, chair of Physical Chemistry II at Ruhr-Universität Bochum (RUB) in the Journal of Physical Chemistry Letters on 18 June 2020.
Insights by THz spectroscopy and simulations
In their study, the researchers investigated the hydrogen bond network around the hydrophobic solvated alcohol tert-butanol, as researchers use alcohols as a prototype models for hydrophobic molecules. The team combined results from terahertz (THz) spectroscopy and simulations.
In THz spectroscopy, researchers measure the absorption of THz radiation in a sample. The absorption spectrum provides a fingerprint of the water network.
They obtained a detailed picture of the water layers surrounding the molecule. "We refer to the innermost layer as HB-wrap, where HB stands for water-hydrogen bond," explains Martina Havenith. The top layer is called HB-hydration2bulk, as it describes the interface to the bulk water. Combined, both layers of the coating are sometimes no thicker than a single layer of water molecules. "Occasionally, a single water molecule may be part of both layers."
Inner layer is longer stable
When the temperature is increased, the outer layer melts first, and the HP-wrap layer remains longer intact. "The inner layer has also less freedom to form distinct configurations due to the hydrophobicity of the solute," says Havenith. "As individual water molecules must always turn away from the alcohol, they form a two-dimensional, loose network." Water molecules in the outer layer have more freedom to move and therefore also more possibilities to connect with other water molecules; researchers refer to this phenomenon as greater entropy.
This type of interaction is relevant for the folding processes of proteins as well as biomolecular recognition between a drug and its target molecule. Understanding the role of water plays a crucial role in the process.
More information: V. Conti Nibali et al. Wrapping Up Hydrophobic Hydration: Locality Matters, The Journal of Physical Chemistry Letters (2020). DOI: 10.1021/acs.jpclett.0c00846
Journal information: Journal of Physical Chemistry Letters
Provided by Ruhr-Universitaet-Bochum