Electrochemical reaction powers new drug discoveries

A Cornell-led collaboration is flipping the switch on traditional synthetic chemistry by using electricity to drive a new chemical reaction that previously stumped chemists who rely on conventional methods.
This new reaction—detailed in the team's paper, "Dual Electrocatalysis Enables Enantioselective Hydrocyanation of Conjugated Alkenes," published June 29 in Nature Chemistry—could spur the manufacture of a host of new, low-cost drugs.
The project was a collaboration between co-senior authors Song Lin and Robert A. DiStasio Jr., both assistant professors of chemistry and chemical biology in the College of Arts and Sciences.
Lin's lab is exploring the potential applications of electrochemistry, which drives chemical reactions with voltage instead of the reagents favored by traditional organic chemistry. Those reagents can be expensive and difficult to control at larger scales. And while electrochemistry is often employed in battery and energy research, it is less commonly used in chemical synthesis.
Lin has been particularly focused on using electrocatalysis to create chiral molecules—molecules that are non-superimposable mirror images of each other (often referred to as left- and right-handed) and quite prevalent throughout medicinal chemistry. For this project, his group partnered with pharmaceutical company Eli Lilly to identify specific reaction transformations that could be targeted to create low-cost precursors for new drugs.
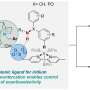
In this work, Lin and his team developed a catalyst that performs asymmetric catalysis—a way of steering chemical reactions towards a specific chiral product (e.g., production of the right-handed version of a chiral molecule instead of the left-handed one).
"This really allowed us to improve the selectivity of the reaction, so you can get a product pure enough to be used, potentially, for drug discovery purposes," Lin said. "While this work does not necessarily change the way the drugs are manufactured, it does provide us with access to a large variety of analogs."
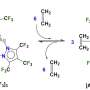
The researchers were able to combine two different reactions—cobalt-mediated hydrogen-atom transfer and copper-promoted radical cyanation—on an alkene substrate using a dual catalytic strategy.
"We have two different catalysts in the system, and each of them takes on a specific role," Lin said. "Electrochemistry allows us to combine these two chemical systems seamlessly, and power multiple chemical cycles or different oxidation events in the same reaction system."
The reaction that resulted—asymmetric hydrocyanation of alkenes—has eluded organic chemists for decades. Now, by varying the substrate, they can tweak the molecular structure of a commercial drug and create new varieties.
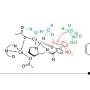
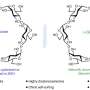
To better understand the mechanism behind this long-sought reaction, Lin turned to DiStasio, whose lab specializes in theoretical chemistry. Particularly relevant is the work DiStasio and his group have done describing and understanding the non-bonded (or non-covalent) interactions that occur between molecules.
"After performing a number of detailed quantum mechanical calculations on this system, it became clear to us that the copper catalyst has a rather interesting and dichotomous nature," DiStasio said. "By combining both attractive and repulsive non-covalent interactions, this catalyst enables a very difficult, yet tremendously useful, chemical reaction."
More information: Lu Song et al. Dual electrocatalysis enables enantioselective hydrocyanation of conjugated alkenes, Nature Chemistry (2020). DOI: 10.1038/s41557-020-0469-5
Journal information: Nature Chemistry
Provided by Cornell University