May 15, 2020 feature
Watching the in situ hydrogen diffusion dynamics in magnesium on the nanoscale
Switchable materials that have extreme material contrast and short switching times with negligible degradation can contribute to active plasmonic and nanophotonic systems. In order to understand their supreme properties, researchers must gather in-depth knowledge about nanoscopic processes. In a new study now published on Science Advances, Julian Karst and a team of scientists at the University of Stuttgart, Germany, investigated nanoscopic details of the phase transition dynamics of metallic magnesium (Mg) to dielectric magnesium hydride (MgH2) using free-standing films to conduct nanoimaging in the lab. The team used characteristic MgH2 phonon resonance to obtain unprecedented chemical specificity between the material states. The results revealed the nucleation process that occurred during nanocrystalline formation. They measured a faster hydride phase propagation at the nanoscale, compared to macroscopic propagation dynamics. The innovative method offers an engineering strategy to overcome limited diffusion coefficients with substantial impact in order to design, develop and analyze switchable phase transition, hydrogen storage and generation materials.
Materials that maintain prominent metal to insulator phase transitions are prime candidates for switchable optical and nanophotonic systems and have undergone extensive research. Such materials can undergo extreme change of optical properties during transition from a metallic to a dielectric phase to form highly relevant switchable optical and active plasmonic systems. In this work, Karst et al. selected Magnesium (Mg) as the archetypical material system, since it has received wide-spread research mainly in the context of hydrogen storage. In its initial metallic state, magnesium is an excellent plasmonic material. When the element is exposed to hydrogen (H2), a phase transition occurs from metallic Mg to the dielectric magnesium (di)hydride (MgH2) to form a highly transparent dielectric material. The MgH2 phase is reversible to the metallic Mg state in a fully cyclic transition. The concept allows researchers to control and reversibly switch the plasmonic resonances of magnesium nanostructures on and off, for applications in switchable metasurfaces (as Mg-to-MgH2), dynamic holography or in plasmonic color displays.
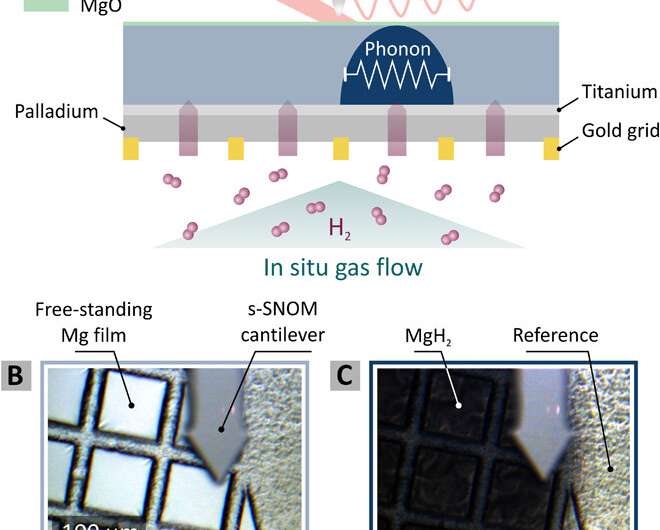
During the experiments, the scientists used gold grids precoated with a 2 to 3-nm film of palladium (Pd). The Pd acted as a catalytic layer to split the hydrogen molecules and enable diffusion into the Mg film. The team used Titanium (Ti) to prevent alloying between Mg and Pd, which could have formed a hydrogen diffusion barrier. In the experimental setup, hydrogen gas accessed the free-standing thin films, while Mg remained accessible for scattering-type scanning near-field optical microscopy (s-SNOM) measurements. Karst et al. scanned the tip of the s-SNOM across the exposed Mg surface to observe and investigate time dynamics of hydride formation and diffusion of hydrogen into the film at nanometer resolution. When they exposed the film to hydrogen concentration in two percent nitrogen (N2), the highly reflective metallic Mg film switched to dielectric MgH2, which appeared black in color.
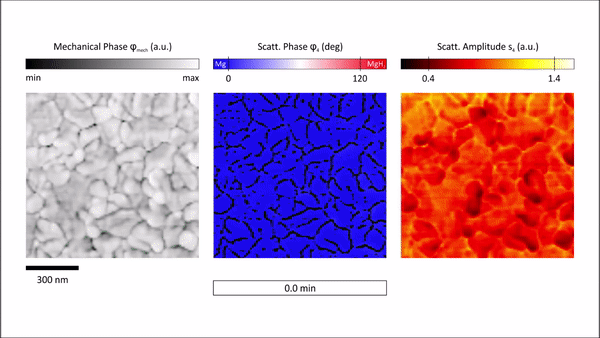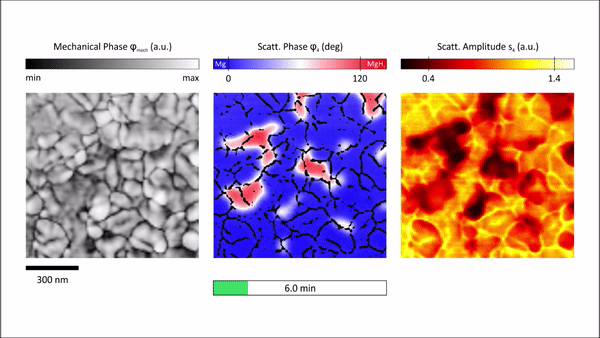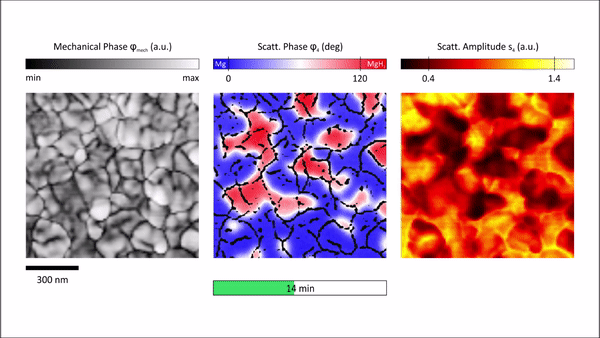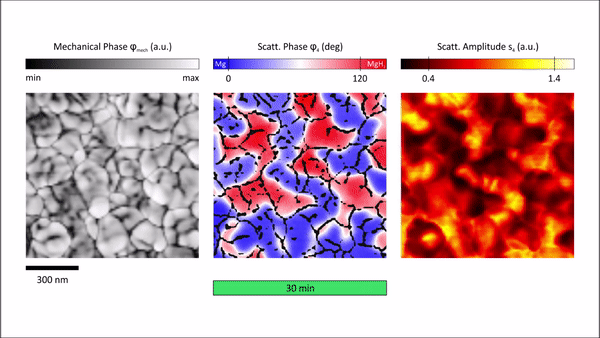
The s-SNOM measurement delivered two main quantities, topological information and information on local optical properties relative to the complex dielectric function. The team then raster scanned the atomic force microscopy cantilever within the s-SNOM setup across the sample surface to deliver surface topography. Demodulation and detection techniques allowed them to obtain information on local properties at nanoscale resolution. In order to probe the local properties of the material, Karst et al. illuminated the tip with a strong light field and noted the scattering amplitude to be influenced by changes in the film topography and local properties. However, the scattering phase detected for Mg (blue) and MgH2 (red) regions showed strong phase contrast due to the characteristic infrared phonon of MgH2, to depict a distinct signature of hydrogenated areas compared to metallic regions. Based on the findings, Karst et al. further studied hydrogenation of free-standing Mg films by inspecting the scattering phase maps by overlaying the phase maps with grain boundary maps to visualize in situ hydrogen absorption in Mg at selected time steps.
-
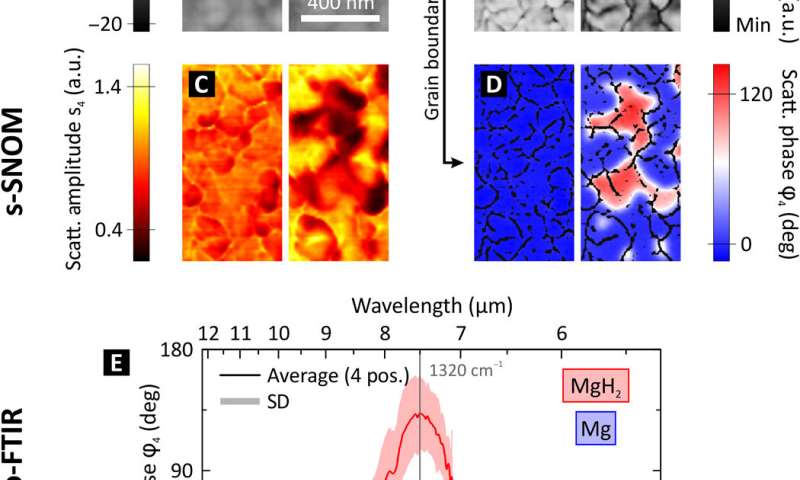
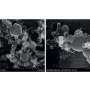
Near-field appearance of the Mg-MgH2 phase transition. (A to D) s-SNOM measurements depicting the same area of a 50 nm Mg film in its pristine state and after 10 min of hydrogenation at room temperature. (A) The topography depicts the expansion of the individual nanocrystallites of the polycrystalline Mg film during hydrogenation. (B) The mechanical phase φmech indicates clear grain boundaries between the individual nanocrystallites of the polycrystalline Mg film. By applying an edge detection filter, we extract a mask of these grain boundaries. (C) The scattering amplitude s4 (fourth demodulation order) drops when metallic Mg changes to dielectric MgH2. However, the scattering amplitude is also highly influenced by the surface roughness, as grain boundaries are visible in the two-dimensional (2D) scans plotted in (C). This leads to an inaccuracy in the determination where Mg has switched to MgH2, as both, a change in the optical properties and a change in the surface morphology/roughness, change the scattering amplitude. (D) The scattering phase φ4 displays a very high material contrast between metallic Mg (blue appearance) and dielectric MgH2 (red appearance). This is achieved by performing s-SNOM measurements at a characteristic IR phonon resonance of MgH2 and allows a chemically specific nanoscale imaging of the hydrogen diffusion without the influence of the surface topography. The 2D images are overlaid with the grain boundary mask from (B). (E) Nano-FTIR spectra of the near-field scattering phase taken on Mg (blue) and MgH2 (red). The plot shows the average and SD of four positions each. The distinct phonon resonance of MgH2 peaks at v¯=1320 cm−1 and causes a maximum scattering phase difference of Δφ ≈ 130° between MgH2 and Mg. Credit: Science Advances, doi: 10.1126/sciadv.aaz0566 -
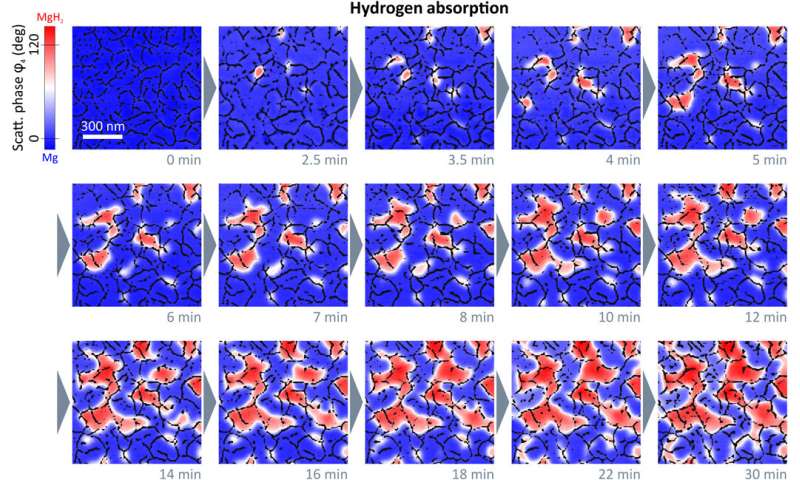
Chemically specific in situ nanoscale imaging of the diffusion dynamics of hydrogen into a 50nm Mg thin film. We plot 2D s-SNOM images of the scattering phase φ4 at several time steps of the hydrogen loading process. All scans are performed with an illumination frequency of v¯=1280 cm−1. Hydrogenated areas (dielectric MgH2) lead to a large shift of the optical phase compared to metallic Mg, as visualized by a blue-to-red transition. An overlay with grain boundary masks allows an excellent tracking of the MgH2 formation and a detailed study of the diffusion mechanism of hydrogen in Mg thin films. We find that hydride formation is nucleated at grain boundaries and is followed by a growth process of these nucleation centers. The hydrogenation front progresses from grain to grain until channels of MgH2 have formed all over the film surface. The phase formation stops, although the surface is not completely switched from Mg to MgH2. Credit: Science Advances, doi: 10.1126/sciadv.aaz0566 -
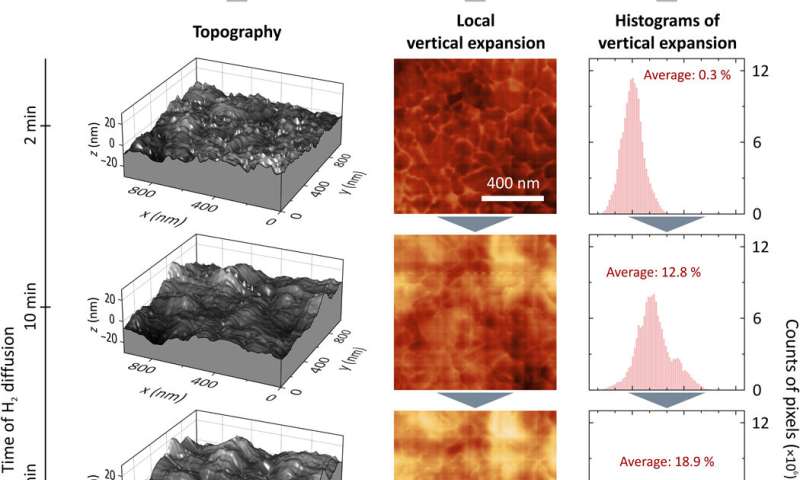
Vertical expansion during hydrogenation. (A) Topography of the Mg thin film after 2, 10, 20, and 60 min of hydrogen exposure. First, there are small peaks appearing. The longer the hydrogenation takes, the rougher/more uneven the surface becomes. (B and C) 2D images of the local vertical expansion and their histograms for the same time steps as in (A) showing a local vertical expansion of more than 60%. The average vertical expansion is calculated by integrating each histogram. (D) Average vertical expansion versus time. For a fully hydrogenated Mg film, one would expect the expansion to be 30%. As the hydrogen absorption in our 50-nm Mg film has saturated while still areas of metallic Mg were left, we reach a maximum average vertical expansion of approximately 25%. This can be explained with the hydrogenation front propagation in the vertical direction through the Mg film. Credit: Science Advances, doi: 10.1126/sciadv.aaz0566
Further analysis allowed the team to distinguish between nanoscopic and macroscopic hydride phase propagation dynamics in Mg to provide insight to hydrogenation at the scale of the individual grain. Hydrogen diffusion in Mg films depended on the morphology of the material. After each individual grain, hydrogenation of the film stopped, allowing for new nucleation before the next grain transformed. However, even after 60 minutes of hydrogenation, the team observed substantial amounts of pristine metallic Mg on the film surface, which contradicted with previous literature on Mg. Karst et al. credited the behavior to several factors, including the blocking layer formed to halt the vertical hydrogen front progression in the setup, which may have left the surface in a pristine state. They also noted the changing film morphology and film expansion on hydrogen exposure as possible contributing factors.
In this way, Julian Karst and colleagues investigated nanoscale hydrogen diffusion dynamics in the lab using s-SNOM. Based on a characteristic IR phonon resonance of MgH2, they allowed chemical specificity to track hydride formation, nucleation and lateral growth. The process was highly influenced by the nanoscale morphology of the Mg film, which was also responsible for the slow diffusion of hydrogen throughout the entire film. The team noted how the process of hydrogenation stopped before the entire film switched, leaving areas of metallic Mg in the dielectric MgH2. The findings have immediate impact for a range of active optical and plasmonic systems using Mg and other transition materials. The work forms an important step forward to enhance and understand the diffusion kinetics, dynamics, and efficiency of phase change across switchable materials.
More information: Julian Karst et al. Watching in situ the hydrogen diffusion dynamics in magnesium on the nanoscale, Science Advances (2020). DOI: 10.1126/sciadv.aaz0566
Ronald Griessen et al. Thermodynamics of the hybrid interaction of hydrogen with palladium nanoparticles, Nature Materials (2015). DOI: 10.1038/nmat4480
M. Wuttig et al. Phase-change materials for non-volatile photonic applications, Nature Photonics (2017). DOI: 10.1038/nphoton.2017.126
Journal information: Science Advances , Nature Photonics , Nature Materials
© 2020 Science X Network