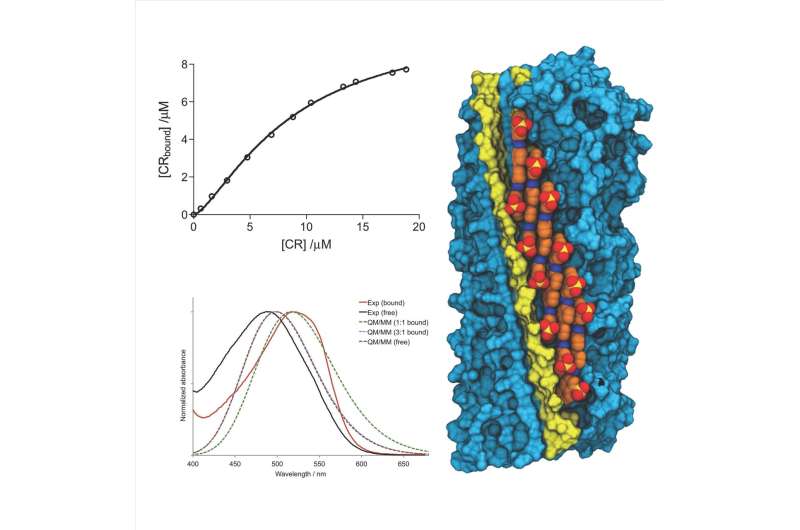
The study focused on the interaction of Congo-Red in amyloid structures formed by the HET-s prion particle. Credit: University of Barcelona
The aggregation of proteins in amyloid structure, a process described in mammals and fungus and bacteria, is implied in about 36 human diseases, including Alzheimer's disease, Parkinson's disease and type 2 diabetes. Most of the amyloid fibres are known for their ability to bind Congo-red, regarded a specific marker of amyloid structures. This adds a great value to the compound Congo-Red, since it is one of the most used to detect the presence of amyloids and characterize the process of aggregation involved in amyloidogenic diseases.
In a paper published in the journal Angewandte Chemistry International Edition, researchers from the Faculty of Pharmacy and Food Sciences, members of the Institute of Nanoscience and Nanotechnology (IN2UB), the Institute of Biomedicine (IBUB) and the Institute of Theoretical and Computational Chemistry (IQTCUB), identified the binding model of this dye. Specifically, the study focused on the interaction of Congo-Red in amyloid structures formed by the HET-s prion particle.
From a technological perspective, the results provide the molecular basis to explain the spectral changes in Congo-Red when it joins amyloid fibres, which enables them to exploit this process in the study of aggregation of other proteins. And most importantly, the fact that knowing this mechanism could enable them to identify potential strategies of inhibition of amyloid fibres involved in amyloid-origin diseases.
More information: Alba Espargaró et al. On the Binding of Congo Red to Amyloid Fibrils, Angewandte Chemie International Edition (2020). DOI: 10.1002/anie.201916630
Journal information: Angewandte Chemie International Edition
Provided by University of Barcelona