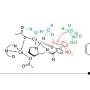
Asymmetric iodoesterification of simple alkenes by concerto catalyst
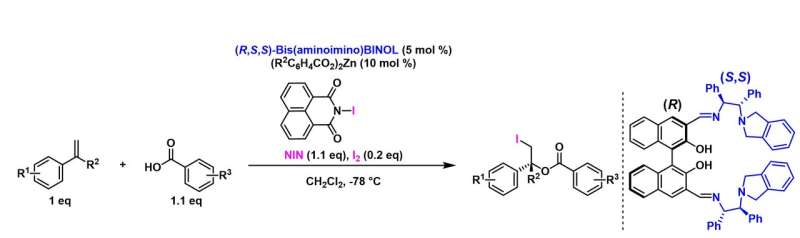
Japanese researchers have succeeded in catalytic asymmetric iodoesterification from simple alkene substrates and carboxylic acids. Published in Angewandte Chemie International Edition on April 27, this new research, was accomplished by precisely controlling multiple interactions in a single catalytic reaction. This synthetic reaction is expected to contribute to the simplification of industrial processes and greater efficiency in optical active drug production.
Catalytic reactions are very important for the process of manufacturing chemical products and pharmaceuticals such as esters and halogen compounds, which are indispensable for everyday life. In particular, when the target compound has a chiral center, it is necessary to selectively obtain the correct optical isomer, and precise catalyst design is essential to the development of an asymmetric catalyst that achieves this. The research reported in the above article efficiently succeeds in obtaining an optically active ester incorporating iodine, and is expected to offer great value and huge potential to industry.
"Among the halogens, iodine is essential for developing and improving the functions of medicines, agrichemicals, and materials," says Professor Takayoshi Arai of Chiba University. "At the same time, the development of a catalyst technology that works to produce high-value-added iodine products is highly significant in Chiba, which is one of the world's leading iodine production sites."
For many years, Professor Arai has succeeded in developing asymmetric catalysts that utilize the properties of various metal complexes, and at the same time has employed a halogen bond as a catalyst design, expected to be capable of selective activation of soft-functionalities with a clear directionality. Professor Arai and his team have been researching catalyst design, and with this latest research, in addition to original catalyst design, they have succeeded in achieving a previously-inaccessible synthetic reaction by collaborating with a theoretical calculation research team.
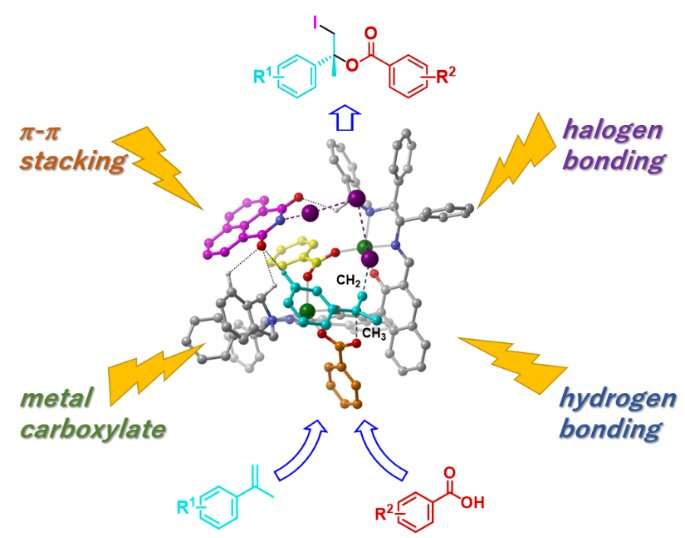
Asymmetric iodolactonization, for which case studies have been reported, can easily be achieved because it involves an intramolecular reaction, but it requires a substrate synthesis and can be used only for particular purposes. By contrast, the iodoesterification reaction is industrially valuable as it combines two different inexpensive and readily available molecules in one reaction. However, in the iodoesterification reaction, both high-level activation and precise recognition of the three-dimensional molecule structure have to be compatible in the intermolecular reaction. Asymmetric iodoesterification reaction could not be realized so far due to the complexity of the reaction.
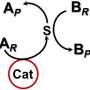
This time, however, the research team developed a new strategy, and they achieved this combined with theoretical calculations in addition to applying the technology and know-how of stereoselective catalysts revealed in previous research. Furthermore, they found that four types of chemical bonds, namely metal carboxylate, halogen bond, hydrogen bond, and π-π stacking, collaborate in catalytic asymmetric iodoesterification by coordinating the formation of one catalyst.
"In this research, we finally succeeded in accomplishing the asymmetric catalytic reaction of iodoesterification for the first time in the world by coordinating four different forces, much like playing a quartet on one catalyst," reports Professor Arai. "We hope that this practical new reaction will contribute significantly to the creation of optically active and highly functional iodine compounds and the industrial application of such compounds."
More information: Takayoshi Arai et al, Catalytic Asymmetric Iodoesterification of Simple Alkenes, Angewandte Chemie International Edition (2020). DOI: 10.1002/anie.202003886
Journal information: Angewandte Chemie International Edition
Provided by Chiba University