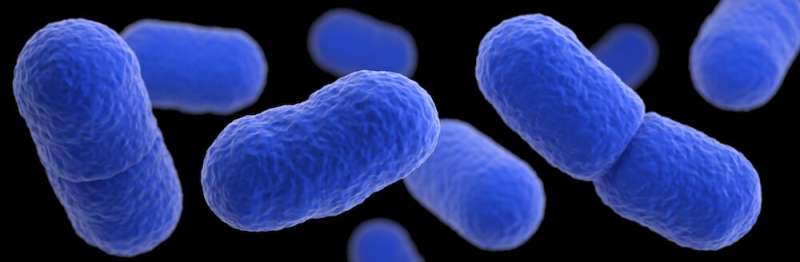
This illustration depicts a 3D, computer-generated image of a grouping of Listeria monocytogenes bacteria. The artistic recreation was based upon scanning electron microscopic imagery. Credit: UTSW
Cells in some of the body's most vulnerable entry routes to bacterial infection buffer themselves when the immune system detects danger by reorganizing the cholesterol on their surfaces, a new study led by UTSW scientists suggests. The findings, published today in Nature Microbiology, could offer new strategies for fighting infections that don't involve antibiotics.
Scientists have long known that the mucus membranes that line the intestines, lungs, and other sites play a key role in protecting the body from systemic infection. But exactly how the immune system enhances the defensive properties of so-called mucosal epithelial cells to block infectious agents, such as bacteria, is unclear, say UT Southwestern Medical Center researchers Neal M. Alto, Ph.D., a professor of microbiology, and Arun Radhakrishnan, Ph.D., an associate professor of molecular genetics.
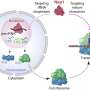
Because immune cells, such as macrophages, are typically found in close proximity to mucosal epithelia, Alto and his colleagues wondered whether these cells might secrete a molecule that helps epithelial cells heighten their defenses when the immune system detects a threat, such as an infectious microbe. To investigate this idea, the researchers grew epithelial cells in a petri dish with activated macrophages, then added Listeria monocytogenes, the bacterial species that causes the foodborne illness known as listeriosis. These epithelial cells were significantly more resistant to infection than those in a dish without the macrophages.
When the researchers broadly surveyed gene activity in the macrophages, they found that one in particular, called cholesterol 25-hydroxylase (CH25H), became significantly more active when confronted with L. monocytogenes. Further tests showed that the small molecule produced by this gene was key for preventing epithelial infection.
This gene was discovered at UTSW a quarter century ago, says Radhakrishnan, in the lab space he now occupies. Because Radhakrishnan's own work focuses on cholesterol metabolism—a process in which CH25H plays a starring role—his lab and Alto's formed a collaboration to better understand how this gene might be strengthening epithelial cells' defenses.
Radhakrishnan explains that CH25H changes cholesterol, which normally doesn't mix at all with water, to produce a form called 25-hydroxycholesterol (25HC) that does slightly mix with water. This property of 25HC is exploited to regulate the amount of cholesterol, an essential lipid in every cell in the body. Some of 25HC's functions include turning down the activity of genes involved in cholesterol synthesis and activating an enzyme that converts cholesterol to a form that can be stored in cells.
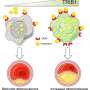
Surprisingly, when the researchers treated epithelial cells with 25HC, they found that total cholesterol in these cells didn't change during the time period of their experiments. However, using two different types of sensor molecules—one that attaches to cholesterol on the cell surface that's accessible, and another that detects cholesterol on the cell surface that's inaccessible because it's bound by other lipids—Alto, Radhakrishnan, and their colleagues discovered that 25HC depletes the accessible cell-surface cholesterol, pulling it inside the cell.
"Within one hour of treatment, the accessible form of cholesterol was severely depleted from the cell surface," says Radhakrishnan. "By four hours, it was completely gone."
The depletion of accessible cholesterol was essential to protect epithelial cells from L. monocytogenes, Alto says, reliably bolstering the cells' defenses. When the scientists treated the depleted cells with an enzyme that converted the inaccessible cholesterol on the cell surface to an accessible form, the cells became susceptible to infection again.
This defense mechanism worked not only against L. monocytogenes but also Shigella flexneri, a bacterial pathogen that causes a disease called shigellosis, highlighting the broadly antimicrobial nature of this protection.
The scientists plan to further study the mechanism behind this phenomenon to potentially identify parts of this pathway that could be controlled or enhanced by pharmaceuticals. They also plan to test whether this protection applies to viral infections as well.
"Exploring this process in greater depth could give us new leads into potentially manipulating cholesterol metabolism as a way of enhancing immunity to pathogens," Alto says.
More information: Oxysterols provide innate immunity to bacterial infection by mobilizing cell surface accessible cholesterol, Nature Microbiology (2020). DOI: 10.1038/s41564-020-0701-5 , nature.com/articles/s41564-020-0701-5
Journal information: Nature Microbiology
Provided by UT Southwestern Medical Center