Reducing sulfur dioxide emissions alone cannot substantially decrease air pollution
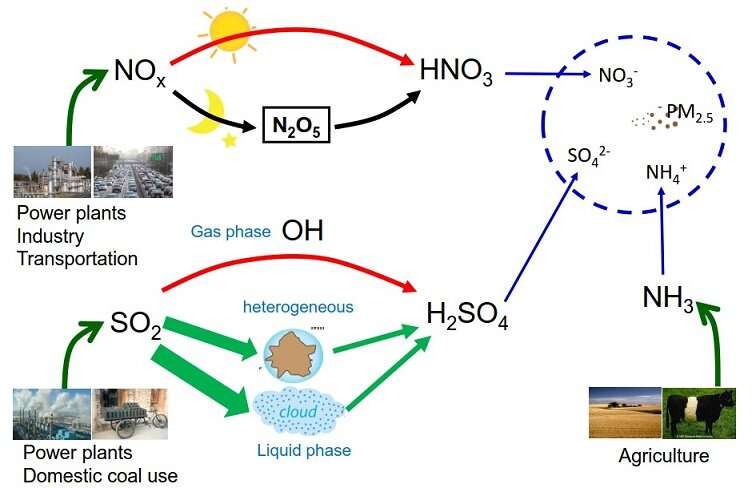
High loadings of fine particulate matter (PM2.5) during haze are mostly produced from the chemical reactions of reactive gas precursors, including sulfur dioxide (SO2), nitrogen oxides (NOx), ammonia (NH3), and volatile organic compounds. In an ideal world, air pollution would be cured by wiping clean any one of these four PM2.5 precursors. However, in the real world, we have to go step by step, considering the technological conditions and the economic costs in the emission control strategies.
These gases are subject to a certain thermodynamic equilibrium in the atmosphere. Theoretically, NH3 prefers to combine with SO2 (sulfuric acid) to form ammonium sulfate, which is stable in the atmosphere. Excessive NH3 will react with nitrogen dioxide (nitric acid) to form ammonium nitrate, which is unstable, and the formation of which is influenced by the relative abundance of NH3 and nitrogen dioxide. Consequently, a decrease in SO2 emissions leaves more NH3 to form ammonium nitrate, and it may also perturb the balance between NH3 and nitrogen dioxide.
Due to the delivery of the Air Pollution Control Action Plan, SO2 emissions have declined dramatically since 2013. It also offers us an opportunity to examine whether a reduction in SO2 will perturb the balance between NH3 and nitrogen dioxide in forming ammonium nitrate, and to decide how to make emission control strategies in the future.
Professor Xingying Zhang from the National Satellite Meteorological Center and his coauthors have addressed this issue. They evaluated and compared the behavior of PM2.5 with respect to NOx and NH3 emission changes in high (2013) and low (2018) SO2 emission cases.
Prof. Zhang's group has found that, from 2013 to 2018, due to the changes in precursor emissions, the simulated annual mean PM2.5 concentration decreased by nearly 20%, more than half of which was driven by reduced SO2 emissions. "To evaluate the influence of a reduction in SO2 emissions on the sensitivity of PM2.5 to NOx and NH3 emissions, we conducted model sensitivity studies by separately perturbing NOx and NH3 emissions by 25%. Then, we calculated the relative reduction of PM2.5 concentration caused by a 1% decrease in NOx and NH3 emissions," explains Professor Zhang.
According to the study of Prof. Zhang, it can be concluded that, due to the reduced emissions of SO2, and considering the high level of NH3 emissions in China, nitrogen dioxide emissions control is more effective in reducing the surface PM2.5 concentration in China. This paper has been published in Atmospheric and Oceanic Science Letters.
More information: Guangyi XU et al, Changes in PM2.5 sensitivity to NOx and NH3 emissions due to a large decrease in SO2 emissions from 2013 to 2018, Atmospheric and Oceanic Science Letters (2020). DOI: 10.1080/16742834.2020.1738009
Provided by Chinese Academy of Sciences