Credit: CC0 Public Domain
New insight on the conditions that control self-assembly in the protective shell of viruses has been published today in eLife.
The study also highlights the factors that can cause incorrect self-assembly in the viral protein shell, otherwise known as the capsid, preventing viruses from being able to replicate. The findings suggest that manipulating these factors to induce misassembly in viral capsids could be a promising new approach to hindering viral infections.
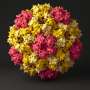
Viruses are formed by a chain of the nucleic acids DNA or RNA that are encased in a protein shell made, in the simplest cases, from multiple copies of a single protein. This capsid protects, carries and delivers viruses to their host. Despite this apparent simplicity in their make-up, viruses are able to perform many complex functions that are essential to their replication cycle—one of these being the ability of the viral capsid to assemble itself. The resulting structure of a correctly self-assembled capsid has a very precise architecture, which in most cases is spherical and similar to an icosahedron, with 20 identical triangular faces.
"During self-assembly, a favourable binding energy competes with the energetic cost of the growing edge and the elastic stresses generated by the curvature of the capsid," explains lead author Carlos Mendoza, a researcher at Universidad Nacional Autónoma de México (the National Autonomous University of Mexico). "As a result, incomplete structures such as open capsids and cylindrical or ribbon-shaped shells may emerge during assembly, preventing the successful replication of viruses."
Mendoza says that previous studies of self-assembly in capsids have mostly focused on the templated growth on the surface of a sphere, or on analysing the optimal shape of the resulting capsid. They have not considered the potential importance of other ingredients on capsid stability and formation, such as the line tension (energy penalty per unit length at the rim of a growing capsid), the chemical potential difference (free-energy gain of the proteins upon assembly) or the preferred curvature.
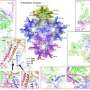
To address this gap, Mendoza and co-author David Reguera, Professor at the University of Barcelona and the UB Institute of Complex Systems, Spain, analysed the conditions and mechanisms leading to the misassembly of empty viral capsids, taking into account all these 'ingredients.' Their analyses revealed that capsid self-assembly depends on three factors that can be manipulated to cause the formation of non-spherical and open shells.
"We found that the outcome of self-assembly can be recast into a universal phase diagram, a type of chart that highlights the conditions for successful viral assembly and the key factors that prevent it," Reguera explains. "Our findings advance our understanding of the physics controlling the assembly of curved shells, and explain why viruses with high mechanical resistance cannot be assembled directly and need a maturation process to stiffen the capsid and become infective."
The authors add that their results can only be applied directly to icosahedral viruses, which include papillomavirus, polyomavirus and poliovirus, and not to viruses with helical nucleocapsids, such as SARS-CoV-2, the virus that causes COVID-19. However, their work lays the foundation for future studies into the conditions and chemical agents needed to hinder different types of viral infections by preventing capsid assembly or by inducing misassembly.
More information: Carlos I Mendoza et al, Shape selection and mis-assembly in viral capsid formation by elastic frustration, eLife (2020). DOI: 10.7554/eLife.52525
Journal information: eLife
Provided by eLife