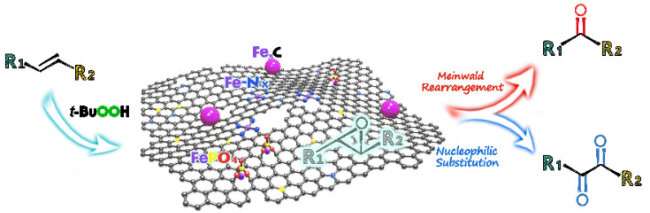
Synthesis of ketones and 1,2-diketones via oxidation of alkenes catalyzed by bifunctional iron nanocomposite catalyst. Credit: SONG Tao
Oxidation of alkenes to carbonyl compounds is one of the most important industrial reactions. The oxidation products are important and useful intermediates or building blocks in synthetic organic chemistry, pharmaceuticals, agrochemicals and bulk/fine chemicals.
Traditional synthetic methods require harsh and environmentally unfriendly conditions. Therefore, developing a green and efficient catalytic process is highly desirable.
Recently, a research team led by Prof. Yang Yong from the Qingdao Institute of Bioenergy and Bioprocess Technology (QIBEBT) of the Chinese Academy of Sciences (CAS) reported the fabrication of a bifunctional iron nanocomposite catalyst, in which two catalytically active sites of oxidation and Lewis acid sites are simultaneously integrated into a porous carbon.
As a bifunctional catalyst, it exhibited high efficiency for direct oxidative cleavage of alkenes into ketones or their oxidation into 1,2-diketones with a broad substrate scope and high functional group tolerance in water under mild reaction conditions. Meanwhile, the catalyst is highly stable and can be recycled for several times.
This work not only opens up a fancy pathway in the rational design of Fe-N-C catalysts, but also offers a simple and efficient method for accessing industrially important ketones and 1,2-diketones from alkenes in a cost-effective and environmentally benign fashion.
The study was published in ACS Catalysis. It was supported by the Key Technology R&D Program of Shandong Province and the Royal Society (UK) for a Newton Advanced Fellowship.
More information: Tao Song et al. A Bifunctional Iron Nanocomposite Catalyst for Efficient Oxidation of Alkenes to Ketones and 1,2-Diketones, ACS Catalysis (2020). DOI: 10.1021/acscatal.9b05197
Journal information: ACS Catalysis
Provided by Chinese Academy of Sciences