April 9, 2020 report
Engineered enzyme able to break down PET in ten hours
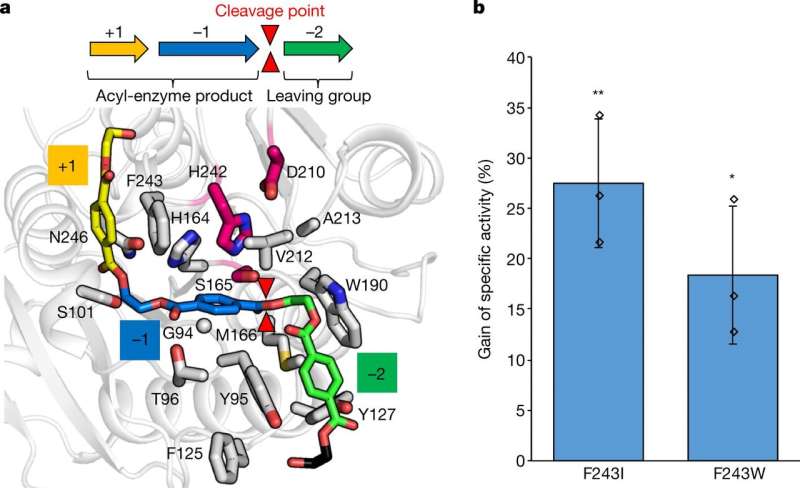
A team of researchers from TBI, Université de Toulouse, CRITT Bio-Industries and Carbios, Biopôle Clermont Limagne, has engineered a commonly known enzyme to efficiently break the chains that hold the building blocks of polyethylene terephthalate (PET) together. In their paper published in the journal Nature, the group describes how they developed the enzyme and how well it worked in a test plant.
PET is a very common type of plastic used in products from soda bottles to plastic bags—it is also the source of a lot of trash. Despite efforts by consumers to recycle such materials, the ability of recyclers to tear them down into their basic parts for reuse is quite limited. Until now, processes that have been used to break the bonds holding PET monomers together have been inefficient—just 30 percent of the materials are reused. In this new effort, the researchers have engineered an enzyme known to break down plastic for higher efficiency.
The leaf-branch compost cutinase enzyme, as its name suggests, is an enzyme found in nature that is able to break the bonds that hold leaves together, making them digestible. Prior research has shown that they are able to do the same with PET, but very inefficiently. The team began their work by taking a closer look at the enzyme looking specifically for the key amino acids that were used to bind to the linkers holding PET monomers together. They then created hundreds of mutant enzymes with different amino acid properties.
Next, they tested the mutants on their plastic munching abilities. After much effort, the were able to find and isolate the mutant that worked best—they found it to be 10,000 times more efficient at cutting PET bonds than the native enzyme. The team then mass-produced batches of the mutant enzyme and placed them in a reactor for testing. They found that the enzyme they had created was capable of breaking down 200 grams of PET in just 10 hours—and that it was 90 percent efficient. They then used the broken-down material generated by the enzyme to create new PET, and found that it was just as strong as those that had been recycled. The team plans to build a larger reactor to prove that the process is economically viable.
More information: V. Tournier et al. An engineered PET depolymerase to break down and recycle plastic bottles, Nature (2020). DOI: 10.1038/s41586-020-2149-4
Journal information: Nature
© 2020 Science X Network




















