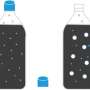
The candy-cola soda geyser experiment, at different altitudes

Dropping Mentos candies into a bottle of soda causes a foamy jet to erupt. Although science fair exhibitors can tell you that this geyser results from rapid degassing of the beverage induced by the candies, the precise means by which bubbles form hasn't been well characterized. Now, researchers reporting in ACS' Journal of Chemical Education used experiments in the lab and at various altitudes to probe the mechanism of bubble nucleation.
During production, soda is carbonated by sealing it under carbon dioxide pressure that is about four times the total air pressure. This causes carbon dioxide to dissolve in the beverage. When someone opens the container, carbon dioxide escapes from the space above the liquid, and the dissolved carbon dioxide slowly enters the gas phase, eventually causing the soda to go "flat." Mentos greatly speed up this process: Carbon dioxide flows into tiny air bubbles on the rough surface of the candies, allowing the gas to rapidly jet to the surface of the soda. Thomas Kuntzleman and Ryan Johnson wondered if atmospheric pressure plays a role in carbon dioxide bubble formation. They reasoned that the answer could reveal more details of the process.
In the lab, the researchers added a Mentos candy to water carbonated at different pressures and measured the mass lost from the liquid over time. They fit these data to an equation that allowed them to estimate that the bubbles on the surface of the candy were about 6 μm in diameter. In contrast to other candies, Mentos could have a fortuitous balance between bubble size and the number of bubble sites that allows them to produce excellent fountains, the researchers suggest. Then, the researchers left the lab and examined the extent of soda foaming after candy addition at different altitudes, ranging from Death Valley (43 feet below sea level) to Pikes Peak (14,108 feet above sea level). They observed increased foam production at higher elevations; however, this effect could not be explained by the simple application of gas laws. Similar experiments could form the basis of classroom projects for students in general science through physical chemistry courses, the researchers say.
More information: Thomas S. Kuntzleman et al. Probing the Mechanism of Bubble Nucleation in and the Effect of Atmospheric Pressure on the Candy–Cola Soda Geyser, Journal of Chemical Education (2020). DOI: 10.1021/acs.jchemed.9b01177
Journal information: Journal of Chemical Education
Provided by American Chemical Society