Two-dimensional metals open pathways to new science
An atomically thin materials platform developed by Penn State researchers in conjunction with Lawrence Berkeley National Lab and Oak Ridge National Lab will open a wide range of new applications in biomolecular sensing, quantum phenomena, catalysis and nonlinear optics.
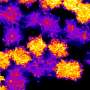
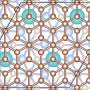
"We have leveraged our understanding of a special type of graphene, dubbed epitaxial graphene, to stabilize unique forms of atomically thin metals," said Natalie Briggs, a doctoral candidate and co-lead author on a paper in the journal Nature Materials. "Interestingly, these atomically thin metals stabilize in structures that are completely different from their bulk versions, and thus have very interesting properties compared to what is expected in bulk metals."
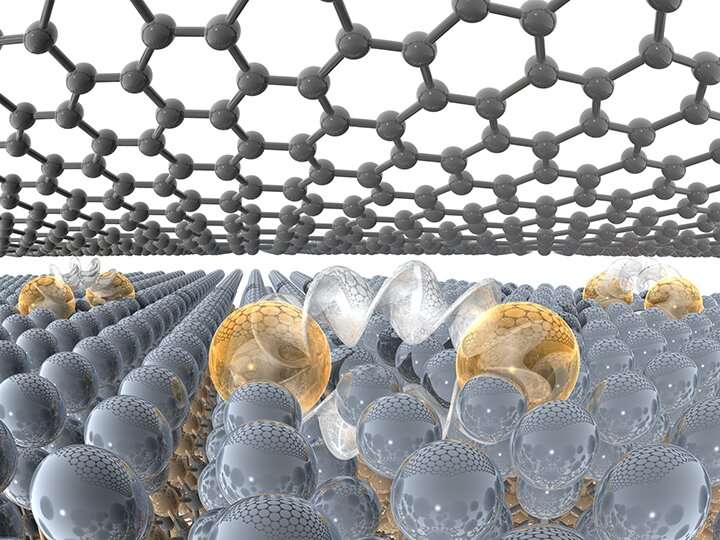
Traditionally, when metals are exposed to air they rapidly begin to oxidize—rust. In as short as one second, metal surfaces can form a rust layer that would destroy the metallic properties. In the case of a 2-D metal, this would be the entire layer. If you were to combine a metal with other 2-D materials via traditional synthesis processes, the chemical reactions during synthesis would ruin the properties of both the metal and layered material. To avoid these reactions, the team exploited a method that automatically caps the 2-D metal with a single layer of graphene while creating the 2-D metal.
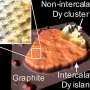
The researchers start with silicon carbide that they heat to a high temperature. The silicon leaves the surface, and the remaining carbon reconstructs into epitaxial graphene. Importantly, the graphene/silicon carbide interface is only partially stable and is readily passivated by nearly any element, if the element has access to this interface.
The team provides this access by poking holes in the graphene with an oxygen plasma, and then they evaporate pure metal powders onto the surface at high temperatures. The metal atoms migrate through the holes in the graphene to the graphene/silicon carbide interface, creating a sandwich structure of silicon carbide, metal and graphene. The process to create the 2-D metals is called confinement heteroepitaxy, or CHet.
"We call it CHet because of the confined nature of the metal, and the fact that it is epitaxial—the atoms all line up—to the silicon carbide, an important aspect to the unique properties we see in these systems," noted Joshua Robinson, senior author and associate professor of materials science and engineering, Penn State.
"In this paper, the focus is on the fundamental properties of the metals that are going to enable a new set of research topics," said Robinson. "It shows that we are able to develop novel 2-D materials systems that are applicable in a variety of hot topics such as quantum, where graphene is a key link that allows us to think about combining very different materials that normally could not be combined to form the basis for superconducting or photonic qubits."
Next steps in their studies will involve proving out the superconducting, sensing, optical and catalytical properties of these layered materials. Beyond creating unique 2-D metals, the team is continuing to explore new 2-D semiconducting materials with CHet that would be of interest to the electronics industry in future electronics beyond silicon.
Additional authors from Penn State include former doctoral student in the Robinson group and co-lead author Brian Bersch, doctoral student Yuanxi Wang, and professors Cui-Zu Chang, Jun Zhu, Adri van Duin and Vincent Crespi.
The Nature Materials paper is "Atomically Thin Half-van der Waals Metals via Confinement Heteroepitaxy."
More information: Atomically thin half-van der Waals metals enabled by confinement heteroepitaxy, DOI: 10.1038/s41563-020-0631-x , nature.com/articles/s41563-020-0631-x
Journal information: Nature Materials
Provided by Pennsylvania State University