Study reveals breast cancer cells shift their metabolic strategy to metastasize
New discovery in breast cancer could lead to better strategies for preventing the spread of cancer cells to other organs in the body, effectively reducing mortality in breast cancer patients.
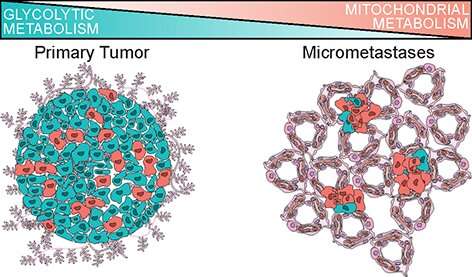
According to a study, published today in Nature Cell Biology, breast cancer cells shift their metabolic strategy in order to metastasize. Instead of cycling sugar (glucose) for energy, they preferentially use mitochondrial metabolism.
"This has important potential clinical implications because it suggests that drugs targeting mitochondrial metabolism may have efficacy for preventing metastatic spread in patients," said Devon A. Lawson, Ph.D., assistant professor in the UCI Department of Physiology and Biophysics and a member of the Chao Family Comprehensive Cancer Center at the UCI School of Medicine. "Historically, tumors were thought to contain dysfunctional mitochondria and be principally sustained by anaerobic glycolysis, or Warburg metabolism. Our work challenges that dogma and shows that breast cancer cells use mitochondrial metabolism during metastatic spread."
Despite major advances in the detection and treatment of early stage disease, metastasis—when cancer cells in the breast spread to other organs in the body—accounts for approximately 40,000 deaths among women in the U.S. each year. It is the number one cause of nearly all mortality associated with breast cancer.
Previous work suggests that metastasis is seeded by rare primary tumor cells with unique biological properties that enable them to spread, causing the cancer to take hold in other locations in the body. While properties promoting cell motility and migration are well studied, mechanisms governing the seeding and establishment of small collections of cancer cells in distal tissues are not. This is in part because metastatic seeding cannot be studied in humans, and because it is technically challenging to detect and analyze rare cells at this transient stage in animal models.
"Through our research, we established a robust new method for identifying global transcriptomic changes in rare metastatic cells during seeding using single-cell RNA-sequencing and patient-derived xenograft (PDX) models of breast cancer," said Ryan Davis, first author on the study and a doctoral student in the Lawson laboratory. "We found that metastatic cells harbor distinct RNA molecules that are highly predictive of poor survival in patients and alter metabolism in a way that can be targeted therapeutically."
More information: Ryan T. Davis et al, Transcriptional diversity and bioenergetic shift in human breast cancer metastasis revealed by single-cell RNA sequencing, Nature Cell Biology (2020). DOI: 10.1038/s41556-020-0477-0
Journal information: Nature Cell Biology
Provided by University of California, Irvine