Method yields high rate of D-lactate using cyanobacteria, could revolutionize bioplastic production
A Kobe University led research team has illuminated the mechanism by which cyanobacteria (Synechocystis sp. PCC 6803) produces D-lactate, showing that malic enzyme facilitates this production. Subsequently, they succeeded in producing the world's highest rate (26.6g/L) of D-lactate directly from CO2 and light by modifying the D-lactate synthesis pathway using genetic engineering .
It is expected that this success will contribute towards the development of important process technologies for producing polylactic acid, which is used to manufacture biodegradable plastics. This could help make the concept of a sustainable, low carbon society a reality.
The research group consisted of Professor HASUNUMA Tomohisa (of Kobe University's Engineering Biology Research Center), Project Associate Professor HIDESE Ryota (of Kobe University's Graduate School of Science, Technology and Innovation) and Associate Professor OSANAI Takashi (of Meiji University's School of Agriculture).
The results of this research were published online in the international scientific journal ACS Synthetic Biology on January 31, 2020.
The utilization of bioproduction to synthesize versatile chemical compounds and functional raw materials that are usually derived from oil is vital for both the environment and resource sustainability. In recent years, bioproduction methods using microbes have gained attention. Among these microbes are microalgae. It is possible to produce various useful substances such as oils and pigments from microalgae using sunlight and CO2.
Cyanobacteria is a type of quick growing microalgae that is easy to genetically modify. Cyanobacteria has been used to produce D-lactate before, however the low yield has been an obstacle which prevents the practical application of these methods.
Cyanobacteria turns CO2 into the sugar glycogen via photosynthesis. If cyanobacteria which has accumulated glycogen inside its cells is then placed in a dark environment with no oxygen, the glycogen is metabolized by the cyanobacteria and it secretes organic acids (such as succinic and lactic) into the growth medium.
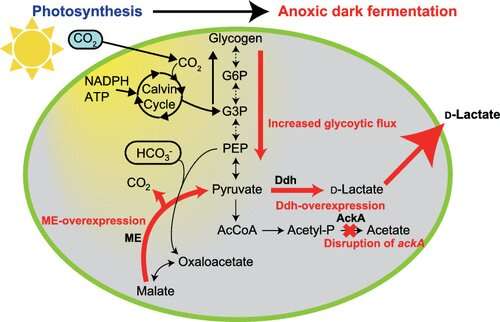
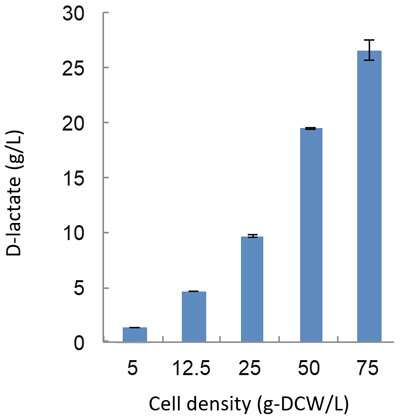
To synthesize D-lactate from cyanobacteria, there needs to be an increase in pyruvate production. This research group discovered that the malic enzyme, which converts malic acid into pyruvate, is vital for D-lactate production. They used dynamic metabolomics to illuminate the mechanism behind D-lactate production. Through this analysis, they discovered that when excessive malic enzyme is produced inside the cells, not only is malic acid converted into pyruvate, but also the pathway to produce pyruvate from glycogen is activated (Figure 1). D-lactate is biosynthesized from pyruvate by D-lactate dehydrogenase. The research group succeeded in producing 26.6 g/L of D-lactate with a conversion rate of 94.3% from the accumulated glycogen by genetically engineering the D-lactate dehydrogenase to optimize its function (Figure 2).
This research represents an important advancement towards the development of an industrial process to produce D-lactate from CO2. The group aims to continue to boost D-lactate production through metabolic pathway optimization and analysis of cultivation conditions.
There is a large market for D-lactate, which can be used as a raw material in the production stereocomplex PLA that is a biodegradable plastic. On the other hand, high purity and productivity are required in order to make biologically synthesizing D-lactate using microbes viable. Bioproduction methodologies which use heterotrophic microbes like E. coli exist, however these use sugar (glucose) from corn or sugarcane as a source of energy for production. This means that growing these plants for bioproduction causes a multitude of issues, such as competition with food sources, use of arable land and freshwater resources, and contribution to environmental destruction (for example, deforestation).
Cyanobacteria, on the other hand, is an ideal microbe for producing useful substances, since it can convert CO2 fixed via photosynthesis into various target compounds. In addition, cyanobacteria has a much higher photosynthesizing ability than plants, meaning that it can even be grown under strong light. It does not require soil and many varieties can be cultivated in seawater. Therefore it is hoped that cyanobacteria can provide the ultimate basis for bioproduction as it only requires sunlight, CO2 and seawater.
It is widely known that cyanobacteria could provide a way to synthesize D-lactate, and attempts have been made to boost D-lactate production using genetic modification. However, almost all systems that produce D-lactate are connected to propagation via photosynthesis so low amounts of this target substance are synthesized. The reason for this is that the mechanism for D-lactate production in cyanobacteria has not been well understood.
Metabolome analysis techniques allow researchers to both identify and calculate the multitude of compounds found inside cells. This research group developed "Dynamic Metabolomics' which allowed them to observe the amount of substances metabolized over time.
The Synechocystis sp. PCC 6803 cyanobacteria used in this study is one of the most commonly researched cyanobacteria worldwide. It is a model organism for photosynthate production because it is easy to genetically modify and grows fast. Previous research conducted by this group using dynamic metabolomics showed that succinic acid is mainly produced via malic acid in Synechocystis sp. PCC 6803. The current study focused on malic enzyme, which converts malic acid into pyruvate. First, they aimed to elucidate the effects of malic enzyme on the metabolism of Synechocystis sp. PCC 6803 through dynamic metabolomics. Their subsequent objective was to increase D-lactate production using metabolic engineering.
Research Methodology
Two types of cell were created in order to comprehensively investigate the mechanism behind D-lactate production: 1. Cells which had no malic enzyme function and 2. Cells in which this function was optimized, leading to an overexpression of malic enzyme.
Dynamic metabolomics was used to analyze the difference in the metabolism between these two cells. It was found that more pyruvate was produced from glycogen when the level of malic acid in the cells was low (Figure 1).
The research group further genetically modified malic enzyme optimized cells to overexpress D-lactate dehydrogenase, and enhanced the D-lactate dehydrogenase's function of producing D-lactate from pyruvate. In addition, the group genetically engineered the cells to remove the enzyme acetate kinase in order to suppress the production of byproduct acids.
The modified Synechocystis sp. PCC 6803 was then cultivated in a dark anoxic environment (fermentation conditions). Under these conditions, the cells reached the optimum density. This research group far exceeded the world's previous highest yield of D-lactate (10.7 g/L), by producing 26.6 g/L at a rate of 0.185 g/L/h (Figure 2). It is thought that this finding can contribute towards a low cost process to produce high levels of D-lactate.
Further Research
Cyanobacteria can be utilized to produce many versatile chemical compounds and functional raw materials, however this technology is not yet well developed enough to be implemented on an industrial scale. The big problem is that lower levels of the target compound are produced using cyanobacteria, compared to the amounts produced when using heterotrophic microorganisms. The current research has shown that the dynamic metabolomics analysis is highly effective for evaluating the function of Synechocystis. Based on the result of the dynamic metabolomics, this group enabled the Synechocystis to perform at their full potential by genetically modifying their metabolism.
It is hoped that increasing the photosynthetic productivity of cyanobacteria through dynamic metabolomics and metabolic engineering could contribute towards the realization of a sustainable, low carbon society.
More information: Ryota Hidese et al. Malic Enzyme Facilitates d-Lactate Production through Increased Pyruvate Supply during Anoxic Dark Fermentation in Synechocystis sp. PCC 6803, ACS Synthetic Biology (2020). DOI: 10.1021/acssynbio.9b00281
Journal information: ACS Synthetic Biology
Provided by Kobe University