Tricky reaction sequence gets a major boost from a flow setup and statistics
Researchers from Osaka University optimize a complicated domino reaction in a flow system via machine learning to efficiently screen multiple variables, attaining high selectivity and yield of a potential biologically active compound
Despite technological advances, early drug discovery and development remain a time-consuming, difficult and inefficient process with low success rates. A team from Osaka University has discovered a possible solution for overcoming low production yields in complex reaction sequences, providing a proof-of-concept study in the successful high yield of a potential therapeutic agent.
In a study recently published in Chemical Communications, the researchers demonstrate the production of a potential drug agent using machine-learning to rapidly screen experimental conditions for a complex reaction series. This optimization approach significantly reduced the time, materials and cost required for conventional methods.
For both academic and industrial researchers, an essential step in the development of chemical reactions involves optimizing experimental conditions. This is traditionally achieved by varying one parameter and keeping the others constant—an onerous and costly process. A strategy for quickly identifying optimal parameters is machine learning, a statistical tool used in many fields, including drug discovery.
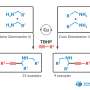
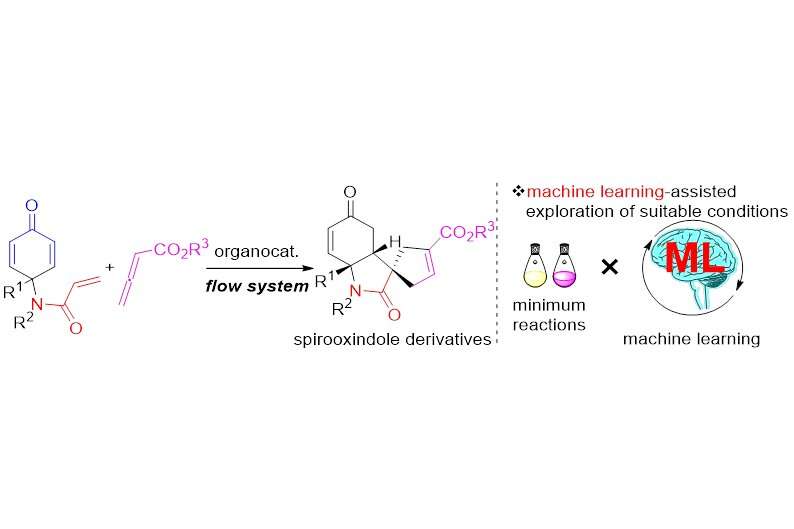
"While examining the steps of the organocatalyzed Rauhut–Currier and [3+2] annulation sequence, we first realized that a micro-mixing flow system would suppress any undesired side reactions and improve the yield of the desired biologically active spirooxindole derivative," says senior author of the study, Hiroaki Sasai. "The Gaussian process regression (GPR) then allowed us to quickly screen different parameters and explore the optimal flow conditions for our system to maximize the product yield."
These spirooxindole motifs, found in many biologically active molecules and natural products, have gained considerable research interest as possible antiviral drug agents. As with other drugs, making spirooxindoles results in mixtures containing mirror-image variants of the same molecule (enantiomers) with different chemical properties (e.g., drug activity vs. no activity)—the tricky part is preferentially maximizing the yield of the desired variant showing drug activity. A simplified method for achieving this feat with spirooxindoles has remained mostly out of reach until now.
Despite the complexity, selectivity and specificity of the highly efficient reaction sequence, the researchers established the reaction using a micro-mixer flow system, albeit with 49% yield. Using the optimized parameters from GPR, they then obtained the spirooxindole derivatives with three contiguous chiral centers within one minute with up to 89% yield and 98% purity of the desired mirror-image variant.
"It is challenging to predict the effect of changing each experimental parameter when developing a novel reaction without a thorough reaction optimization," explains lead author Masaru Kondo. "However, combining tools like GPR with new synthetic methods in flow systems may simplify and streamline the drug development process for other complicated molecules, reducing cost, time and material waste."
More information: Masaru Kondo et al. Exploration of flow reaction conditions using machine-learning for enantioselective organocatalyzed Rauhut–Currier and [3+2] annulation sequence, Chemical Communications (2019). DOI: 10.1039/C9CC08526B
Journal information: Chemical Communications
Provided by Osaka University