Manipulating surface tension in fluids to suit various industrial needs
What do dishwashing liquid and nonstick pans have in common? Besides the fact that one is used to clean the other after cooking, both household items derive their usefulness by affecting the physical properties of fluids and the surfaces those fluids come into contact with.
Dishwashing liquid strips oil from surfaces because its chemical makeup consists of one water repellent (hydrophobic) group which binds preferentially to oil, and one 'water-loving' (hydrophilic) group which binds preferentially to water. This effectively reduces the surface tension of oil, allowing it to be rinsed off easily.
On the other hand, non-stick pans are typically coated with a material known as polytetrafluoroethylene—better known as Teflon—which is extremely hydrophobic. Virtually anything slides off Teflon-coated surfaces because the adhesive forces between Teflon and many other materials or molecules are almost negligible.
Surfactants like dishwashing liquid and materials like Teflon have other uses beyond mundane household chores. In fact, they are involved in a whole range of industrial processes, including the extraction of crude oil and the prevention of fouling in biomedical devices. To meet such diverse industrial needs, A*STAR scientists are constantly exploring novel materials and techniques for modifying how fluids interact with each other and with surfaces.
Clues from nature
While human ingenuity has been the source of many synthetic methods to control the way oil and water interact, nature is often a greater inventor, as Nanji Hadia, a Research Scientist at A*STAR's Institute of Chemical and Engineering Sciences (ICES), discovered.
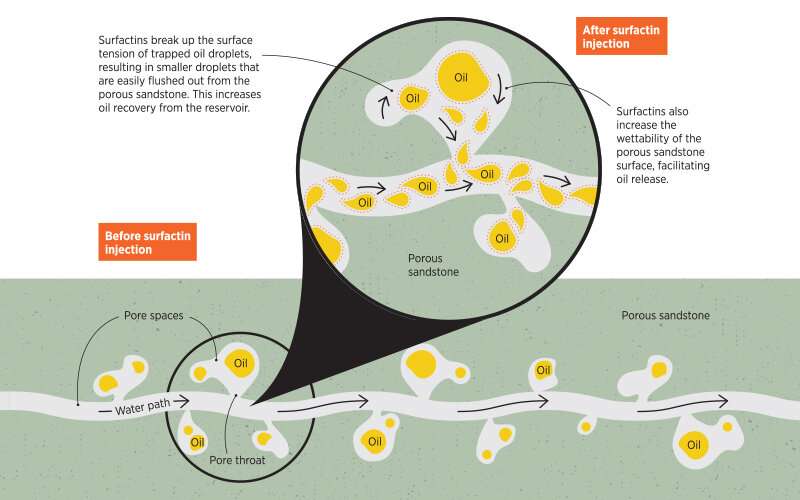
Working on the problem of crude oil extraction, Hadia was looking for more environmentally friendly ways to recover residual oil in reservoirs. "In most oil reservoirs, a large amount of oil remains unrecoverable because it is stuck in the micron-scale pore throats of reservoir rocks," said Hadia. "Capillary forces are a key factor which holds back the oil in these tiny pore throats."
To maximize the amount of crude oil that can be recovered from each oil well, the oil industry has developed an approach known as enhanced oil recovery. "One type of enhanced oil recovery involves the use of surfactants to overcome the capillary forces holding the oil in place and allow oil and water to mix into emulsions which can then flow more easily through the pores," Hadia said.
However, many existing synthetic surfactants include sulfates and sulfonates which are not always biodegradable and pose environmental concerns. "Because of increasingly stringent environmental restrictions, it is a priority for the petroleum exploration and production industry to move towards green surfactants," Hadia noted.
Hadia therefore decided to take a leaf out of nature's innovation handbook, collaborating with his colleague Christoph Ottenheim to produce natural surfactant-like molecules from bacteria, also called biosurfactants. Two types of biosurfactants have been widely studied in the context of enhanced oil recovery: glycolipids, which are fat molecules with a sugar chain attached to them; and lipopeptides, fat molecules with a short chain of amino acids (the basic building blocks of proteins) attached to them.
Taking tension away
In their study, Hadia and Ottenheim focused on a lipopeptide called surfactin, produced by the Bacillus family of bacteria. "Surfactins are known for their ability to drastically reduce the interfacial tension between oil and water," Hadia said. But producing surfactins in sufficiently large amounts without incurring astronomical costs remains a difficult problem to solve and is a major consideration for the oil production industry.
Currently, surfactin is obtained by fermentation, whereby Bacillus are provided with a specific mix of nutrients and kept under precise pH, temperature and aeration conditions that favor surfactin synthesis. "In our case, we used Bacillus subtilis 22.2 strains and allowed them to ferment a nutrient broth overnight at 30°C," Ottenheim explained. The fermentation product contained a mixture of surfactins, with a yield below 1 g/L of broth.
Evaluating the effect of their surfactin mixture on two stock tank crude oils, the researchers found that a low surfactin concentration of 0.025% was able to reduce the interfacial tension of both oils by a hundred to a thousand-fold, thereby facilitating the formation of oil-in-water emulsions. This in turn allows oil to flow easily from pore throats.

To better mimic the real-world conditions under which the surfactin mixture would have to perform, Hadia's team also carried out coreflooding experiments by trapping oil in Berea sandstone, a natural porous sandstone material, then trying to recover the oil. "We were able to recover 1.5-5% more oil by injecting 0.1% surfactin solution into the rock samples [as compared to when surfactin was not used]," Hadia said.
Part of this improvement could also be attributed to the fact that surfactin changed the wettability of the rock samples to make them more water-wet (in the same way that dishwashing liquid works), allowing the oil to be flushed out with water. Taken together, surfactins represent a feasible and environmentally friendlier solution for enhanced oil recovery in the petroleum industry, Hadia noted.
A contact sport
The ability to tune surface wettability is just as useful outside the field of oil recovery. For example, in microfluidics, which involves liquids passing through very tiny channels, the wettability of those channels significantly influences how liquids flow inside them, or whether biological cells can adhere to the channels' surfaces for organ-on-chip purposes.
Instead of applying a coating (like Teflon or surfactin), another strategy to modify surface wettability is through the use of lasers. Researchers led by Zhongke Wang, a Research Scientist at A*STAR's Singapore Institute of Manufacturing Technology (SIMTech), have used laser irradiation to precisely control the wettability of polycarbonate, a type of plastic that is transparent and biocompatible.
"So far, most of the existing techniques can only increase or decrease the wettability of polycarbonate, but not tune the wettability both ways," Wang said, adding that current methods also lack the flexibility to create arbitrary contact angles between polycarbonate surfaces and liquids. A contact angle of more than 90° means that a surface is hydrophobic, whereas a contact angle of less than 90° indicates that a surface is hydrophilic. In other words, a hydrophilic surface has a higher wettability than a hydrophobic one.
Using a femtosecond laser, so called because it emits ultrashort pulses of focused light, Wang's team was able to make polycarbonate surfaces hydrophobic or hydrophilic to varying extents, depending on parameters such as laser intensity, the number of laser pulses applied and the scanning speed of the laser.
For example, a high scanning speed of 0.5 mm/s with a pulse number of 60 resulted in a contact angle of more than 150° between polycarbonate and a 0.5-mm water droplet, which means that the polycarbonate became superhydrophobic. On the other hand, a lower scanning speed of 29 mm/s with fewer pulses resulted in a contact angle of less than 5°, indicative of a superhydrophilic surface.
"The changes in chemical bonds on the polycarbonate surface as a result of different laser irradiation conditions determine the wettability of the laser-scanned surface. Polar groups induced by laser irradiation resulted in a hydrophilic surface, while non-polar groups induced by laser irradiation resulted in a hydrophobic surface," Wang explained.
Seeking synergies
Having demonstrated that the polycarbonate surface is chemically and physically altered by the femtosecond laser, the researchers wanted to test the stability of the surface properties in the context of microfluidics. In microfluidics, ultrasonication—the application of highfrequency sound waves—is commonly used to rinse out the tiny channels in the presence of water or chemicals.
Wang's team found that the roughness of hydrophobic surfaces was increased by ultrasonication with water and ethanol. In contrast, the roughness of hydrophilic surfaces was decreased. The researchers also noted that the hydrophobicity and hydrophilicity of the laser-treated polycarbonate surfaces decreased upon ultrasonication with water and ethanol.
"We think that there are two possibilities for the change in surface wettability post-treatment: ultrasonication could have dislodged debris from the laser-treated surfaces, or ultrasonication may have resulted in additional changes to the chemical bonds on the polycarbonate surface," Wang explained. He added that future experiments using X-ray photoelectron spectroscopy will be needed to clarify whether changes to the chemical bonds have indeed occurred after ultrasonication.
The findings from Wang's work may have implications for Hadia's research since microfluidics is useful for flow visualization on a very small scale. "In the case of enhanced oil recovery studies, microfluidics helps researchers understand the displacement of one fluid by another in a glass microfluidic chip containing a pore network that represents a porous rock sample," Hadia said. Hence, while Wang and Hadia may be studying different problems, their research fields subtly overlap and generate synergies.
Both scientists also offer different approaches to surface modification, be it through chemical means (coating with surfactin) or physical methods (lasers). Their discoveries pave the way for greater efficiency and reduced environmental impact of industrial processes, exemplifying how research and development can produce nifty solutions for some of society's most challenging problems.