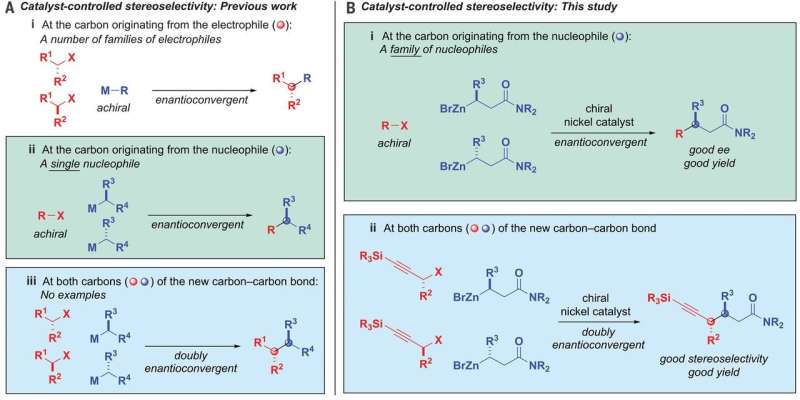
Alkyl-alkyl bond formation. (A) Catalyst-controlled stereoselectivity—previous work. (B) Catalyst-controlled stereoselectivity—this study. ee, enantiomeric excess; M, metal; R, substituent; X, leaving group. Credit: Science (2020). DOI: 10.1126/science.aaz3855
A team of researchers at California Institute of Technology has found a nickel catalyst that bonds alkyl nucleophile and alkyl electrophiles to make a single stereoisomer with two chiral centers. In their paper published in the journal Science, the group describes their process. Jianyu Xu and Mary Watson with the University of Delaware have published a Perspective piece on the work done by the team in the same journal issue.
In chemistry, reactions that result in materials with carbon-carbon bonds are desirable because they are used in large-scale applications such as pharmaceuticals and agriculture products. Such reactions typically rely on transition metal-based catalysts—but there are limitations that prevent many from being used. Many only work with alkyl compounds, which can lead to undesirable side reactions. Also, many such reactions result in racemic mixtures of products, resulting in inefficiencies (racemic mixtures are those that have equal amounts of left and right-handed enantiomers of a chiral molecule).
Linking racemic mixtures of two alkyls while maintaining control of the stereochemistry of both ends of the product has historically been considered to be very difficult. But now, that has changed, thanks to the work by the team at CIT, and that has led to a method for carrying out reactions that lead to desirable carbon-carbon bonded materials.
In their work, the team developed a nickel catalyst that allows an alkyl nucleophile to bond with an alkyl electrophile. Notably, both are racemic carbon compounds. They used a ligand that was bidentate—that allowed open spots on the nickel to bind with the oxygen on the nucleophiles.
The reaction also involved the use of β-zincated amide nucleophiles and propargylic halide electrophiles. And it was controlled by the catalyst. In overriding chiral information in both of the starting products and then bringing them back together in a predefined way, the carbon-carbon bonds were formed. The result was a single stereoisomer that had two chiral centers. In using the nickel catalyst, the group coupled a racemic mixture with nucleophiles and electrophiles with up to 95 percent stereoselectivity and 82 percent yield. Testing also showed that it was compatible with 19 functional groups.
More information: Haohua Huo et al. Catalyst-controlled doubly enantioconvergent coupling of racemic alkyl nucleophiles and electrophiles, Science (2020). DOI: 10.1126/science.aaz3855
Journal information: Science
© 2020 Science X Network