Chemists discover the mechanism of radiation instability of lithium tetraborate
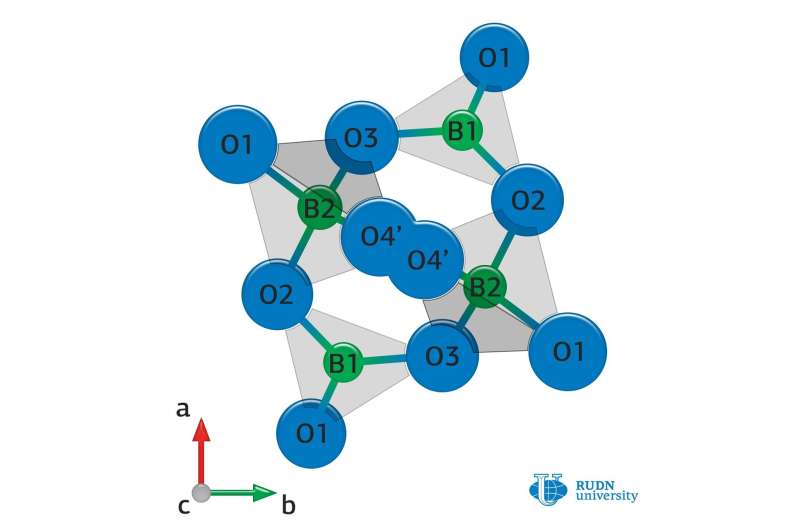
Chemists from RUDN University have studied the mechanism of radiation instability of thermoluminophores based on lithium tetraborate, which are used for the manufacture of radiation dosimeters. They found that the properties of the materials deteriorate due to the breakdown of chemical bonds in the boron-oxygen structure and the formation of clusters of manganese, which was added to lithium tetraborate so that it could exhibit its properties. The work was published in the journal Radiation Measurements.
Lithium tetraborate comprised the first material thermoluminescent radiation dosimeter, highly sensitive to X-ray, gamma and beta radiation. When ionizing radiation enters a thermoluminescent dosimeter, it "stores" the absorbed energy due to the jump of electrons to higher energy levels. When heated above a certain temperature, the electrons emit previously absorbed energy, and the dosimeter begins to glow. The light intensity is proportional to the amount of absorbed radiation.
In order to make lithium tetraborate capable of this, impurities of manganese, silver or other metals are introduced into it, which act as traps for those electrons that were excited by ionizing radiation. But because of these impurities, the radiation resistance of the substance decreases. It has not been known why until now.
RUDN University chemist Alexander Zubov and his colleagues compared ceramic samples based on lithium tetraborate with impurities of manganese, copper, zinc, tin and beryllium. It turned out that the radiation stability of the substance is deteriorating due to the rupture of chemical bonds in the boron-oxygen structure. And while the boron-oxygen lattice in a pure substance is capable of restoring itself during heating, the introduction of manganese interferes with this process.
The more evenly manganese is distributed in the structure of lithium tetraborate, the less negative impact it has on the radiation stability of the material. Copper and tin prevent the clustering of manganese, forming bound complexes with it, thereby preventing it from "migrating" and "sticking" to the crystal lattice during recharging of the dosimeter. Moreover, ceramics with addition of tin, unlike of copper, also have thermoluminescent properties that allow for effective use in dosimetry.
Understanding of the physicochemical processes that occur during irradiation of a material is necessary to create new radiation-resistant materials. The RUDN University chemists were able not only to explain the mechanism of radiation destruction of lithium tetraborate, but also to apply the new knowledge to create a material with a better composition, which can later be used in advanced pocket radiation dosimeters. In addition, the authors argue that their experimental approach, which involves detection of clustered manganese in the structure of lithium tetraborate, can be used as a new effective way to certify the radiation resistance of thermoluminescent dosimeters.
More information: M.I. Danilkin et al. Manganese agglomeration and radiation damage in doped Li2B4O7, Radiation Measurements (2019). DOI: 10.1016/j.radmeas.2019.106134
Provided by RUDN University