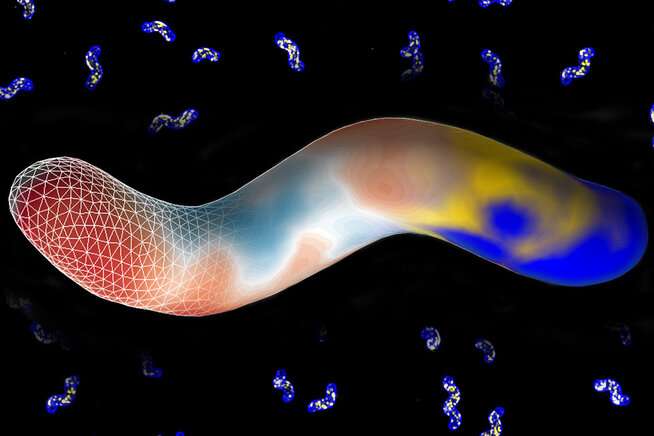
To determine where H. pylori cells add new wall components, the team distinguished between areas of positive curvature. In the background are microscope images of H. pylori cells with new wall components glowing blue and a cell wall-directing protein in yellow. In the foreground is an image of an idealized H. pylori cell showing the transition from the microscopy images (in blue and yellow on the right) to the curvature (in red and blue in the middle). The triangular meshwork on the left represents the mathematically idealized cell surface. Credit: Jenny Taylor
Stomach cancer is the third-leading cause of cancer-related death across the globe. One of the main risk factors for this disease is infection with the bacterium Helicobacter pylori. About half of the world's population is chronically infected with the bacterium, which burrows its way into the mucus lining the stomach and sets up long-term inflammation that can trigger ulcers and, more rarely, cancer.
One of H. pylori's defining characteristics is right there in its name: its helical shape. Its shape doesn't just help scientists spot H. pylori under the microscope; the bug's corkscrew form is critical to its ability to escape stomach acid and anchor in for the long haul by swimming through the stomach's mucus lining.
The bacterium's shape is dictated by the shape of its cell wall, a strong, flexible meshwork that contains its cellular contents. But how does H. pylori make itself helical? It's a fundamental question with practical applications: many antibiotics, including penicillin, interfere with the integrity of bacterial cell walls. A deeper understanding of how different bacterial species pattern themselves could provide the insights needed to develop better-targeted antibiotics.
In new work published in the open-access journal eLife, scientists at Fred Hutchinson Cancer Research Center revealed that H. pylori maintains its helical shape by targeting cell-wall synthesis to two areas with opposite curvature properties. They identified two proteins, MreB and CcmA, that balance cell-wall production in the right areas.
"We're really excited about the fact that we're starting to understand the molecular biophysical mechanism by which H. pylori maintains its helical shape," said Dr. Nina Salama, a bacteriologist at Fred Hutch who led the study. Her group was the first to demonstrate how important H. pylori's corkscrew shape is to its ability to infect its host.
Though a tiny organism, H. pylori is still very large with respect to the molecules that set its shape pattern, she said: "I feel like we're starting to get a handle on how you go from individual proteins to this large macro-molecular structure."
Shape is essential
Unlike our cells, which are encased by a flexible, fatty layer that allows a fair amount of squish, bacteria are encased by cell walls. The cell wall is made up of a single, enormous molecule called peptidoglycan, in which proteins and sugars link up to create a strong, somewhat rigid, but still-flexible mesh that surrounds the bacterium's contents. The cell wall helps contain these contents—and keep bacteria alive. Damage to the cell wall turns the bacterium's insides into outsides: "Basically the cell explodes and dies," Salama said.
Because it's so essential, "cell-wall composition is widely conserved among bacteria," she said. The conservation, or lack of variation, in cell-wall components "means that [the cell wall] is one of the molecular patterns that is recognized by our innate immune system. And since it's so important for maintaining integrity of the bacterial cell, it's a frequent target of antibiotics."
Jenny Taylor, the graduate student in Salama's lab who spearheaded the work, pointed out that while bacterial shape may not top many people's lists of must-answer conundrums, it probably ought to: it's clearly very important to bacteria. They put a lot of effort into maintaining their shapes, which aren't merely the product of bacterial fashion.
In the case of H. pylori, its shape is key to its success as a pathogen. Work by Salama's group showed that in head-to-head competition, helix-shaped H. pylori are clear winners at colonizing the stomach when compared to H. pylori that have been genetically manipulated so that they're shaped like straight rods. A deeper understanding of how the bacterium creates and maintains its shape even as it grows and divides could spur the development of more cell-wall attacking antibiotics.
New visualization tools give new insights
Peptidoglycan, or PG, is created through a complex process in which sugars and protein components are joined together. PG subunits are comprised of two sugars joined to a short protein (or peptide) stem. These are made inside the cell and transported to PG wall, where the sugars link to sugars already in the wall to form long sugar filaments while the peptide stems reach across to join parallel sugar filaments, extending the PG mesh as the bacterial cell grows.
H. pylori cells grow lengthwise before dividing, severing one cell into two midway along the original cell's length. During these processes, the PG macromolecule needs to retain H. pylori's characteristic shape. How H. pylori cells determine where to add more PG subunits while growing is still a largely open question, said the researchers.
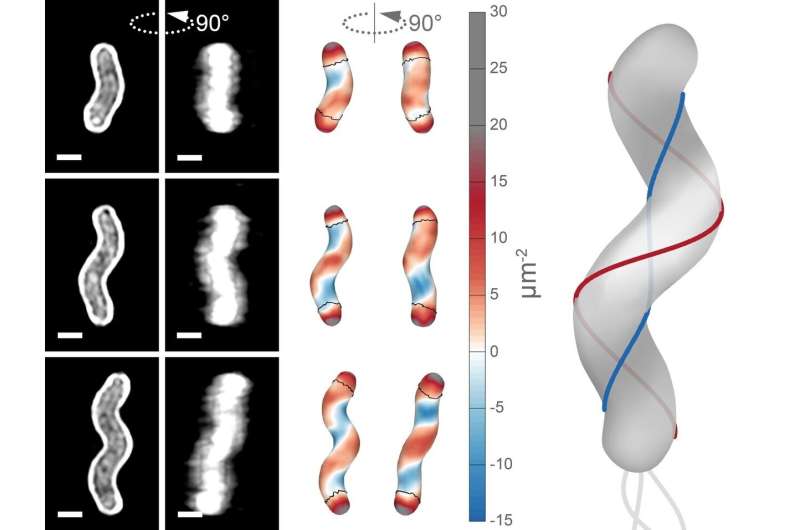
In order to study this process, Taylor worked closely with collaborators at Princeton University, Drs. Joshua Shaevitz and Benjamin Bratton. They needed to lay a lot of technical groundwork, including selecting the best way to use cell-wall curvature to determine where in the cell wall new components were being added. Taylor identified the curvature that characterized what she termed the helix's minor axis, or the shortest helical path between the cell's two ends (show in blue in the figure). The major axis, or the longest helical path (shown in red) has positive curvature. The minor path hugs an interior curve with negative curvature, while the major path traces an exterior curve.
Taylor used fluorescent molecules that could be incorporated into PG, either in the sugar or protein constituents, to tag where new PG synthesis occurred. She used a special type of microscopy, called structured illumination microscopy, to capture the glowing images in layers and computational methods to put these layers together to recreate the cells in three dimensions. This strategy was a big step forward, Salama said, as all previous work had been conducted on 2-D images of bacterial cells. The 3-D imaging allowed Taylor to get a better sense of the surface area where cell-wall components were being added.
On the left are microscopy images of H. pylori cells, and the same cells rotated 90 degrees. In the middle are illustrated bacterial cells showing negative (blue) and positive (red) curvature. On the right is a large illustration of a bacterial cell with the short helical axis (along the negative curvature) traced in blue and the long helical axis (along the positive curvature) traced in red. The grey lines on the left represent flagella, which the bacteria use to swim through stomach mucus. Credit: Salama Lab
Balance maintains the helix
Using the glowing PG-synthesis tags, Taylor looked at where new PG was being added to the cell walls. There were two main possibilities for this pattern: either PG would be added uniformly along the cell wall, or it would be added more in some areas and less in others. She saw that more new cell wall components were being added along both axes compared to areas in between. Although they'd considered the possibility that wall synthesis wouldn't be uniform, they weren't expecting to see it clustered along the shorter minor axis and the longer major axis.
"This was a really surprising result," Taylor said. "Our most intuitive hypothesis was if there is heterogeneity with some synthesis, that we would see an increase [in PG incorporation] at the major axis because there's more area there."
Adding new components to the shorter axis seemed like a recipe for quickly straightening the helix into a rod—but something else about the wall-synthesis process prevents this. Other bacteria use strategies that limit cell-wall building at these areas, but H. pylori takes a different tack. To figure out what that tack is, Taylor looked at two proteins linked to wall building in other bacteria. MreB, which rod-shaped bacteria use to direct wall-building to areas of negative curvature, is thought help straighten out divots in the cell wall. H. pylori relies on the other, CcmA, to maintain its helix. H. pylori cells with defective CcmA achieve only a gentle curve.
Taylor found that in H. pylori, MreB localizes to the negative curvature of the minor axis. CcmA appears to work to balance out MreB's wall-building activities. It strongly prefers the positive curvature of H. pylori's major axis on the opposite side of the cell. In cells with defective CcmA, cell-wall synthesis along the major axis is reduced.
"This is what we would expect if CcmA is helping to drive this synthesis at the major axis," Taylor said.
The findings are consistent with H. pylori using CcmA to build enough cell wall along the major axis that it stays ahead of the cell wall being built along the minor axis, and maintains its helical shape, the researchers said.
Additionally, Taylor showed that H. pylori's major axis is 70% longer than its minor axis.
"That's one of the things that we actually measured for the first time in this paper," Salama said. Theoretically, in a helix, the major axis should be longer, but exactly how much longer hadn't previously been known for H. pylori.
Interplay between creation and destruction
There are still many questions about how H. pylori patterns its cell wall that remain unanswered.
"Cell shape is a summation of all of these activities. It's what you're adding in, what you're taking out, what the structure is at various areas," Taylor noted. She and Salama focused on cell-wall synthesis, though targeted removal of PG units could also help enforce the helical shape. The interplay between creation and destruction, how cell-wall-patterning proteins move around the bacterial cell, as well as exactly how CcmA promotes cell-wall synthesis, remain to be explored.
They'd also like to explore more deeply how cell shape contributes to H. pylori's ability to colonize and survive in the stomach. Is it most important early on, when the bacterium is putting down roots, or does it play a role in helping the bug maintain the chronic infection?
Understanding how specific bacteria maintain their shape could have practical applications, Salama said. Antibiotics are already used to help prevent stomach cancer and ulcers by wiping out H. pylori—but they have downsides.
"Some of these antibiotics that target the cell wall wipe out lots of different bugs," Salama said. "Whereas something that is specific for a bug like Helicobacter, which has this special shape … could [in theory] result in a less-broad, more-specific antibiotic."
More information: Jennifer A Taylor et al. Distinct cytoskeletal proteins define zones of enhanced cell wall synthesis in Helicobacter pylori, eLife (2020). DOI: 10.7554/eLife.52482
Journal information: eLife
Provided by Fred Hutchinson Cancer Research Center