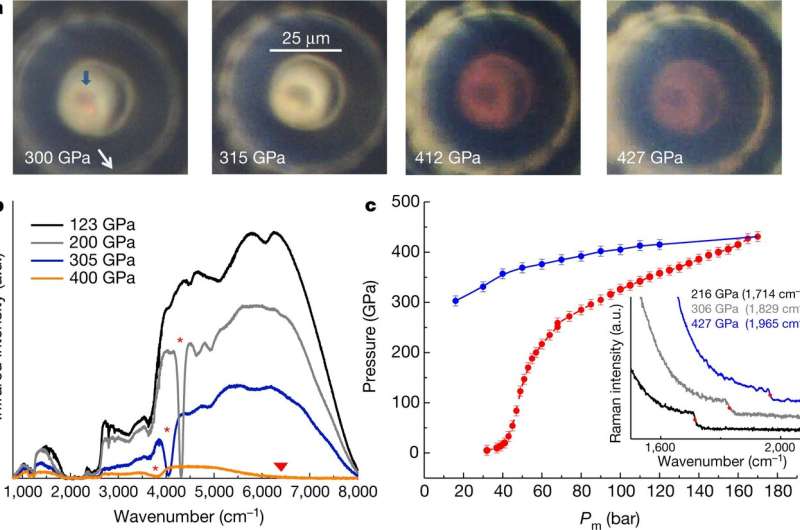
A selection of measurements over the investigated pressure range. a, Photographs of the hydrogen sample taken at different stages of compression, under simultaneous front and back bright-light illumination. The hydrogen sample is indicated by the blue arrow. Around 310 GPa, the sample reversibly turns black, as illustrated by the photographs taken at 315 GPa for the increasing pressure path and at 300 GPa for the decreasing pressure path. At 427 GPa, the sample is in the metallic state and is still distinguishable from the rhenium gasket. The red-coloured aspect at the diamond tip centre is attributed to the decrease of the diamond bandgap. b, Infrared transmission spectra at various pressures. Intrinsic absorption features associated with the vibron and with the closing of the bandgap are indicated by the red stars and the triangle, respectively. c, Pressure evolution in hydrogen versus the helium membrane pressure acting on the piston of the T-DAC, during pressure increase (red) and decrease (blue). Inset, the high-wavenumber part of the Raman diamond spectra collected at three pressures. The wavenumber at the step used to calculate pressure is indicated as a red dot, and noted in the key. Solid lines are guides to the eye. a.u., arbitrary units. Credit: Nature (2020). DOI: 10.1038/s41586-019-1927-3
A team of researchers, two with the French Atomic Energy Commission (AEC) and a third with the Soleil synchrotron, have found evidence of a phase change for hydrogen at a pressure of 425 gigapascals. In their paper published in the journal Nature, Paul Loubeyre, Florent Occelli and Paul Dumas describe testing hydrogen at such a high pressure and what they learned from it.
Researchers long ago theorized that if hydrogen gas were exposed to enough pressure, it would transition into a metal. But the theories were not able to derive how much pressure is required. Doubts about the theories began to arise when scientists developed tools capable of exerting the high pressures that were believed necessary to squeeze hydrogen into a metal. Theorists simply moved the number higher.
In the past several years, however, theorists have come to a consensus—their math showed that hydrogen should transition at approximately 425 gigapascals—but a way to generate that much pressure did not exist. Then, last year, a team at the AEC improved on the diamond anvil cell, which for years has been used to create intense pressure in experiments. In a diamond anvil cell, two opposing diamonds are used to compress a sample between highly polished tips—the pressure generated is typically measured using a reference material. With the new design, called a toroidal diamond anvil cell, the tip was made into a donut shape with a grooved dome. When in use, the dome deforms but does not break at high pressures. With the new design, the researchers were able to exert pressures up to 600 GPa. That still left the problem of how to test a sample of hydrogen as it was being squeezed. The researchers overcame this challenge by simply shining a beam of infrared light down through the center of the device—at normal temperatures, it can pass right through hydrogen. But if it were to meet with a transitioned metal, it would instead be blocked or reflected.
The researchers found that hydrogen samples compressed to 425 gigapascals blocked all infrared and visible light and showed optical reflectivity, as well. They suggest their results indicate that hydrogen does become a solid at 425 gigapascals—but they are already planning another test to bolster their findings. They want to repeat the experiment to determine if the sample begins conducting electricity at 425 gigapascals.
More information: Paul Loubeyre et al. Synchrotron infrared spectroscopic evidence of the probable transition to metal hydrogen, Nature (2020). DOI: 10.1038/s41586-019-1927-3
Journal information: Nature
© 2020 Science X Network